Mouse model of muscleblind-like 1 overexpression: skeletal muscle effects and therapeutic promise
- PMID: 22846424
- PMCID: PMC3471398
- DOI: 10.1093/hmg/dds306
Mouse model of muscleblind-like 1 overexpression: skeletal muscle effects and therapeutic promise
Abstract
Myotonic dystrophy (DM) is a multisystemic disease caused by CTG or CCTG expansion mutations. There is strong evidence that DM1 CUG and DM2 CCUG expansion transcripts sequester muscleblind-like (MBNL) proteins and that loss of MBNL function causes alternative splicing abnormalities that contribute to disease. Because MBNL1 loss is thought to play an important role in disease and localized AAV delivery of MBNL1 partially rescues skeletal muscle pathology in DM mice, there is strong interest in MBNL1 overexpression as a therapeutic strategy. We developed the first transgenic MBNL1 overexpression mouse model (MBNL1-OE) to test the safety and efficacy of multisystemic MBNL1 overexpression. First, we demonstrate that MBNL1 overexpression is generally well-tolerated in skeletal muscle. Second, we show the surprising result that premature shifts in alternative splicing of MBNL1-regulated genes in multiple organ systems are compatible with life and do not cause embryonic lethality. Third, we show for the first time that early and long-term MBNL1 overexpression prevents CUG-induced myotonia, myopathy and alternative splicing abnormalities in DM1 mice. In summary, MBNL1 overexpression may be a valuable strategy for treating the skeletal muscle features of DM.
Figures

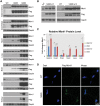
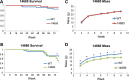
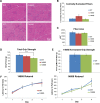

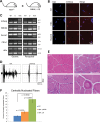
References
-
- Harper P.S. Myotonic Dystrophy. 3rd edn. London: W.B. Saunders; 2001.
-
- Liquori C.L., Ricker K., Moseley M.L., Jacobsen J.F., Kress W., Naylor S.L., Day J.W., Ranum L.P. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–867. - PubMed
-
- Brook J.D., McCurrach M.E., Harley H.G., Buckler A.J., Church D., Aburatani H., Hunter K., Stanton V.P., Thirion J.P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;69:385. - PubMed
-
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–1255. - PubMed
-
- Fu Y.H., Pizzuti A., Fenwick R.G., King J., Rajnarayan S., Dunne P.W., Dubel J., Nasser G.A., Ashizawa T., de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

