Gene duplication, genome duplication, and the functional diversification of vertebrate globins
- PMID: 22846683
- PMCID: PMC4306229
- DOI: 10.1016/j.ympev.2012.07.013
Gene duplication, genome duplication, and the functional diversification of vertebrate globins
Abstract
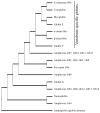
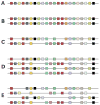
The functional diversification of the vertebrate globin gene superfamily provides an especially vivid illustration of the role of gene duplication and whole-genome duplication in promoting evolutionary innovation. For example, key globin proteins that evolved specialized functions in various aspects of oxidative metabolism and oxygen signaling pathways (hemoglobin [Hb], myoglobin [Mb], and cytoglobin [Cygb]) trace their origins to two whole-genome duplication events in the stem lineage of vertebrates. The retention of the proto-Hb and Mb genes in the ancestor of jawed vertebrates permitted a physiological division of labor between the oxygen-carrier function of Hb and the oxygen-storage function of Mb. In the Hb gene lineage, a subsequent tandem gene duplication gave rise to the proto α- and β-globin genes, which permitted the formation of multimeric Hbs composed of unlike subunits (α(2)β(2)). The evolution of this heteromeric quaternary structure was central to the emergence of Hb as a specialized oxygen-transport protein because it provided a mechanism for cooperative oxygen-binding and allosteric regulatory control. Subsequent rounds of duplication and divergence have produced diverse repertoires of α- and β-like globin genes that are ontogenetically regulated such that functionally distinct Hb isoforms are expressed during different stages of prenatal development and postnatal life. In the ancestor of jawless fishes, the proto Mb and Hb genes appear to have been secondarily lost, and the Cygb homolog evolved a specialized respiratory function in blood-oxygen transport. Phylogenetic and comparative genomic analyses of the vertebrate globin gene superfamily have revealed numerous instances in which paralogous globins have convergently evolved similar expression patterns and/or similar functional specializations in different organismal lineages.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




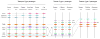
References
-
- Abi-Rached L, Gilles A, Shiina T, Pontarotti P, Inoko H. Evidence of en bloc duplication in vertebrate genomes. Nat Genet. 2002;31:100–105. - PubMed
-
- Alev C, Shinmyozu K, McIntyre BAS, Sheng G. Genomic organization of zebra finch α and β globin genes and their expression in primitive and definitive blood in comparison with globins in chicken. Dev Genes Evol. 2009;219:353–360. - PubMed
-
- Awenius C, Hankeln T, Burmester T. Neuroglobins from the zebrafish Danio rerio and the pufferfish Tetraodon nigroviridis. Biochem Biophys Res Commun. 2001;287:418–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

