Application of microorganisms towards synthesis of chiral terpenoid derivatives
- PMID: 22846902
- PMCID: PMC3427490
- DOI: 10.1007/s00253-012-4304-9
Application of microorganisms towards synthesis of chiral terpenoid derivatives
Abstract
Biotransformations are a standard tool of green chemistry and thus are following the rules of sustainable development. In this article, we describe the most common types of reactions conducted by microorganisms applied towards synthesis of chiral terpenoid derivatives. Potential applications of obtained products in various areas of industry and agriculture are shown. We also describe biological activity of presented compounds. Stereoselective hydroxylation, epoxidation, Baeyer-Villiger oxidation, stereo- and enantioselective reduction of ketones, and various kinetic resolutions carried out by bacteria and fungi have been reviewed. Mechanistic considerations regarding chemical and enzymatic reactions are presented. We also briefly describe modern approaches towards enhancing desired enzymatic activity in order to apply modified biocatalysts as an efficient tool and green alternative to chemical catalysts used in industry.
Figures

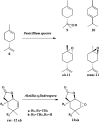
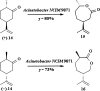
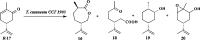

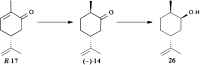



References
-
- Alphand V, Furstoss R, Pedragosa-Moreau S, Roberts SM, Willetts AJ. Comparison of microbiologically and enzymatically mediated Baeyer–Villiger oxidation: synthesis of optically active caprolactones. J Chem Soc Perkin Trans. 1996;1:1867–1872. doi: 10.1039/p19960001867. - DOI
-
- Anderson RJ, Adams KG, Chinn HR, Henrick CA. Synthesis of the optical isomers of 3-methyl-6-isopropenyl-9-decen-1-yl acetate, a component of the California red scale pheromone. J Org Chem. 1980;45:2229–2236. doi: 10.1021/jo01299a038. - DOI
-
- Asakawa Y, Takahashi H, Toyota M, Noma Y. Biotransformation of monoterpenoids, (−)- and (+)-menthols, terpinolene and carvotanacetone by Aspergillus species. Phytochemistry. 1991;30(12):3978–3981.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

