Early stabilizing alveolar ventilation prevents acute respiratory distress syndrome: a novel timing-based ventilatory intervention to avert lung injury
- PMID: 22846945
- PMCID: PMC3521044
- DOI: 10.1097/TA.0b013e31825c7a82
Early stabilizing alveolar ventilation prevents acute respiratory distress syndrome: a novel timing-based ventilatory intervention to avert lung injury
Abstract
Background: Established acute respiratory distress syndrome (ARDS) is often refractory to treatment. Clinical trials have demonstrated modest treatment effects, and mortality remains high. Ventilator strategies must be developed to prevent ARDS.
Hypothesis: Early ventilatory intervention will block progression to ARDS if the ventilator mode (1) maintains alveolar stability and (2) reduces pulmonary edema formation.
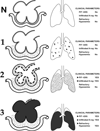
Methods: Yorkshire pigs (38-45 kg) were anesthetized and subjected to a "two-hit" ischemia-reperfusion and peritoneal sepsis. After injury, animals were randomized into two groups: early preventative ventilation (airway pressure release ventilation [APRV]) versus nonpreventative ventilation (NPV) and followed for 48 hours. All animals received anesthesia, antibiotics, and fluid or vasopressor therapy as per the Surviving Sepsis Campaign. Titrated for optimal alveolar stability were the following ventilation parameters: (1) NPV group--tidal volume, 10 mL/kg + positive end-expiratory pressure - 5 cm/H2O volume-cycled mode; (2) APRV group--tidal volume, 10 to 15 mL/kg; high pressure, low pressure, time duration of inspiration (Thigh), and time duration of release phase (Tlow). Physiological data and plasma were collected throughout the 48-hour study period, followed by BAL and necropsy.
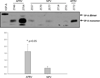
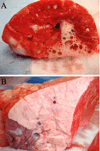
Results: APRV prevented the development of ARDS (p < 0.001 vs. NPV) by PaO₂/FIO₂ ratio. Quantitative histological scoring showed that APRV prevented lung tissue injury (p < 0.001 vs. NPV). Bronchoalveolar lavage fluid showed that APRV lowered total protein and interleukin 6 while preserving surfactant proteins A and B (p < 0.05 vs. NPV). APRV significantly lowered lung water (p < 0.001 vs. NPV). Plasma interleukin 6 concentrations were similar between groups.
Conclusion: Early preventative mechanical ventilation with APRV blocked ARDS development, preserved surfactant proteins, and reduced pulmonary inflammation and edema despite systemic inflammation similar to NPV. These data suggest that early preventative ventilation strategies stabilizing alveoli and reducing pulmonary edema can attenuate ARDS after ischemia-reperfusion and sepsis.
Figures





References
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. - PubMed
-
- Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. - PubMed
-
- McIntyre RC, Jr, Pulido EJ, Bensard DD, Shames BD, Abraham E. Thirty years of clinical trials in acute respiratory distress syndrome. Crit Care Med. 2000;28(9):3314–3331. - PubMed
-
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

