Reconstitution of the very short patch repair pathway from Escherichia coli
- PMID: 22846989
- PMCID: PMC3463329
- DOI: 10.1074/jbc.M112.384321
Reconstitution of the very short patch repair pathway from Escherichia coli
Abstract
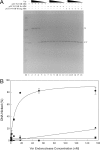
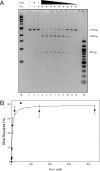
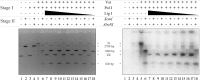
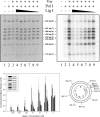
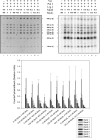
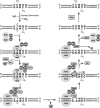
The Escherichia coli very short patch (VSP) repair pathway corrects thymidine-guanine mismatches that result from spontaneous hydrolytic deamination damage of 5-methyl cytosine. The VSP repair pathway requires the Vsr endonuclease, DNA polymerase I, a DNA ligase, MutS, and MutL to function at peak efficiency. The biochemical roles of most of these proteins in the VSP repair pathway have been studied extensively. However, these proteins have not been studied together in the context of VSP repair in an in vitro system. Using purified components of the VSP repair system in a reconstitution reaction, we have begun to develop an understanding of the role played by each of these proteins in the VSP repair pathway and have gained insights into their interactions. In this report we demonstrate an in vitro reconstitution of the VSP repair pathway using a plasmid DNA substrate. Surprisingly, the repair track length can be modulated by the concentration of DNA ligase. We propose roles for MutL and MutS in coordination of this repair pathway.
Figures


References
-
- Harfe B. D., Jinks-Robertson S. (2000) DNA mismatch repair and genetic instability. Annu. Rev. Genet. 34, 359–399 - PubMed
-
- Iyer R. R., Pluciennik A., Burdett V., Modrich P. L. (2006) DNA mismatch repair. Functions and mechanisms. Chem. Rev. 106, 302–323 - PubMed
-
- Jiricny J. (2006) The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 7, 335–346 - PubMed
-
- Schofield M. J., Hsieh P. (2003) DNA mismatch repair. Molecular mechanisms and biological function. Annu. Rev. Microbiol. 57, 579–608 - PubMed
-
- Kunkel T. A., Erie D. A. (2005) DNA mismatch repair. Annu. Rev. Biochem. 74, 681–710 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

