Nuclear-to-cytoplasmic relocalization of the proliferating cell nuclear antigen (PCNA) during differentiation involves a chromosome region maintenance 1 (CRM1)-dependent export and is a prerequisite for PCNA antiapoptotic activity in mature neutrophils
- PMID: 22846997
- PMCID: PMC3460476
- DOI: 10.1074/jbc.M112.367839
Nuclear-to-cytoplasmic relocalization of the proliferating cell nuclear antigen (PCNA) during differentiation involves a chromosome region maintenance 1 (CRM1)-dependent export and is a prerequisite for PCNA antiapoptotic activity in mature neutrophils
Abstract
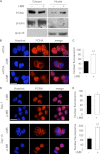
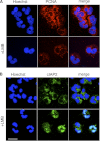
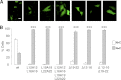
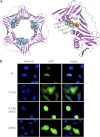
Neutrophils are deprived of proliferative capacity and have a tightly controlled lifespan to avoid their persistence at the site of injury. We have recently described that the proliferating cell nuclear antigen (PCNA), a nuclear factor involved in DNA replication and repair of proliferating cells, is a key regulator of neutrophil survival. In neutrophils, PCNA was localized exclusively in the cytoplasm due to its nuclear-to-cytoplasmic relocalization during granulocytic differentiation. We showed here that leptomycin B, an inhibitor of the chromosome region maintenance 1 (CRM1) exportin, inhibited PCNA relocalization during granulocytic differentiation of HL-60 and NB4 promyelocytic cell lines and of human CD34(+) primary cells. Using enhanced green fluorescent protein fusion constructs, we have demonstrated that PCNA relocalization involved a nuclear export signal (NES) located from Ile-11 to Ile-23 in the PCNA sequence. However, this NES, located at the inner face of the PCNA trimer, was not functional in wild-type PCNA, but instead, was fully active and leptomycin B-sensitive in the monomeric PCNAY114A mutant. To test whether a defect in PCNA cytoplasmic relocalization would affect its antiapoptotic activity in mature neutrophils, a chimeric PCNA fused with the SV40 nuclear localization sequence (NLS) was generated to preclude its cytoplasmic localization. As expected, neutrophil-differentiated PLB985 cells expressing ectopic SV40NLS-PCNA had an increased nuclear PCNA as compared with cells expressing wild-type PCNA. Accordingly, the nuclear PCNA mutant did not show any antiapoptotic activity as compared with wild-type PCNA. Nuclear-to-cytoplasmic relocalization that occurred during myeloid differentiation is essential for PCNA antiapoptotic activity in mature neutrophils and is dependent on the newly identified monomerization-dependent PCNA NES.
Figures



References
-
- Borregaard N. (2010) Neutrophils, from marrow to microbes. Immunity 33, 657–670 - PubMed
-
- Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. (2000) Neutrophils: molecules, functions, and pathophysiological aspects. Lab. Invest. 80, 617–653 - PubMed
-
- Simon H. U. (2003) Neutrophil apoptosis pathways and their modifications in inflammation. Immunol. Rev. 193, 101–110 - PubMed
-
- Rossi A. G., Sawatzky D. A., Walker A., Ward C., Sheldrake T. A., Riley N. A., Caldicott A., Martinez-Losa M., Walker T. R., Duffin R., Gray M., Crescenzi E., Martin M. C., Brady H. J., Savill J. S., Dransfield I., Haslett C. (2006) Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12, 1056–1064 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

