Trans-infection but not infection from within endosomal compartments after cell-to-cell HIV-1 transfer to CD4+ T cells
- PMID: 22846998
- PMCID: PMC3442533
- DOI: 10.1074/jbc.M112.343293
Trans-infection but not infection from within endosomal compartments after cell-to-cell HIV-1 transfer to CD4+ T cells
Abstract
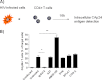
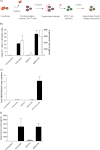
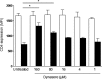
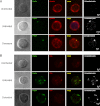
Cellular contacts between HIV-1-infected donor cells and uninfected primary CD4(+) T lymphocytes lead to virus transfer into endosomes. Recent evidence suggests that HIV particles may fuse with endosomal membranes to initiate a productive infection. To explore the role of endocytosis in the entry and replication of HIV, we evaluated the infectivity of transferred HIV particles in a cell-to-cell culture model of virus transmission. Endocytosed virus led to productive infection of cells, except when cells were cultured in the presence of the anti-gp120 mAb IgGb12, an agent that blocks virus attachment to CD4, suggesting that endocytosed virus was recycled to the outer cell surface. Confocal microscopy confirmed the colocalization of internalized virus antigen and the endosomal marker dynamin. Additionally, virus transfer, fusion, or productive infection was not blocked by dynasore, dynamin-dependent endosome-scission inhibitor, at subtoxic concentrations, suggesting that the early capture of virus into intracellular compartments did not depend on endosomal maturation. Our results suggest that endocytosis is not a mechanism of infection of primary CD4 T cells, but may serve as a reservoir capable of inducing trans-infection of cells after the release of HIV particles to the extracellular environment.
Figures


Similar articles
-
HIV-1 entry in SupT1-R5, CEM-ss, and primary CD4+ T cells occurs at the plasma membrane and does not require endocytosis.J Virol. 2014 Dec;88(24):13956-70. doi: 10.1128/JVI.01543-14. Epub 2014 Sep 24. J Virol. 2014. PMID: 25253335 Free PMC article.
-
Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion.Retrovirology. 2011 Dec 6;8:99. doi: 10.1186/1742-4690-8-99. Retrovirology. 2011. PMID: 22145853 Free PMC article.
-
High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells.J Biol Chem. 2004 Dec 3;279(49):51305-14. doi: 10.1074/jbc.M408547200. Epub 2004 Sep 13. J Biol Chem. 2004. PMID: 15371410
-
Endocytosis of HIV: anything goes.Trends Microbiol. 2010 Dec;18(12):543-51. doi: 10.1016/j.tim.2010.09.003. Epub 2010 Oct 19. Trends Microbiol. 2010. PMID: 20965729 Review.
-
HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV.J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. J Transl Med. 2011. PMID: 21284901 Free PMC article. Review.
Cited by
-
Characterization of the influence of mediator complex in HIV-1 transcription.J Biol Chem. 2014 Oct 3;289(40):27665-76. doi: 10.1074/jbc.M114.570341. Epub 2014 Aug 6. J Biol Chem. 2014. PMID: 25100719 Free PMC article.
-
HIV-1 Fusion with CD4+ T cells Is Promoted by Proteins Involved in Endocytosis and Intracellular Membrane Trafficking.Viruses. 2019 Jan 25;11(2):100. doi: 10.3390/v11020100. Viruses. 2019. PMID: 30691001 Free PMC article.
-
Dynasore disrupts trafficking of herpes simplex virus proteins.J Virol. 2015 Jul;89(13):6673-84. doi: 10.1128/JVI.00636-15. Epub 2015 Apr 15. J Virol. 2015. PMID: 25878109 Free PMC article.
-
Exosomes: Implications in HIV-1 Pathogenesis.Viruses. 2015 Jul 20;7(7):4093-118. doi: 10.3390/v7072810. Viruses. 2015. PMID: 26205405 Free PMC article. Review.
-
Fusion of mature HIV-1 particles leads to complete release of a gag-GFP-based content marker and raises the intraviral pH.PLoS One. 2013 Aug 9;8(8):e71002. doi: 10.1371/journal.pone.0071002. eCollection 2013. PLoS One. 2013. PMID: 23951066 Free PMC article.
References
-
- Sattentau Q. (2008) Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6, 815–826 - PubMed
-
- Sigal A., Kim J. T., Balazs A. B., Dekel E., Mayo A., Milo R., Baltimore D. (2011) Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 477, 95–98 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

