eyeGENE(R): a novel approach to combine clinical testing and researching genetic ocular disease
- PMID: 22847030
- PMCID: PMC3553426
- DOI: 10.1097/ICU.0b013e32835715c9
eyeGENE(R): a novel approach to combine clinical testing and researching genetic ocular disease
Abstract
Purpose of review: Molecular genetics is revolutionizing the diagnosis and treatment of inherited eye diseases. The National Eye Institute of the National Institutes of Health (NIH), in an effort to facilitate future basic and clinical research in inherited eye disease, created The National Ophthalmic Disease Genotyping and Phenotyping Network (eyeGENE). This review describes the process and utility of the eyeGENE program as it relates to ophthalmic clinical practice.
Recent findings: Over the last few years, genetic testing of specific genes associated with inherited eye conditions is becoming the standard practice. Vision research and human clinical trials relying on molecular genetic testing of individuals with inherited eye conditions are becoming more common. Eye healthcare professionals must consider the options to assist patients in obtaining genetic testing results and locating trials or studies that may have benefit.
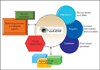
Summary: eyeGENE is a DNA repository and patient registry for inherited eye diseases coupled to phenotypic descriptors and molecular genetic information. Through eyeGENE, healthcare professionals throughout the United States and Canada can obtain Clinical Laboratory Improvement Amendments-certified clinical molecular genetic results on their patients. Researchers may request access to a de-identified database of phenotype and genotype information about eyeGENE participants and DNA aliquots for their research studies. eyeGENE also offers participants the option of being included in a patient registry, whereby they may be re-contacted if an approved clinical study for which they might qualify is offered.
Conflict of interest statement
All authors work for the National Eye Institute at the National Institutes of Health and are involved in operations of eyeGENE.
Figures
References
-
- Bainbridge J, Ali R. Success in sight: the eyes have it! Ocular gene therapy trials for LCA look promising. Gene Therapy. 2008;15:1191–1192. - PubMed
-
-
Simons D, Boye S, Hauswirth W, et al. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet–Biedl syndrome mouse model. Proc Natl Acad Sci USA. 2011;108:6276–6281. This study demonstrates successful gene therapy in Bbs4 knockout mice through a subretinal injection of the wild-type gene complement incorporated into a viral vector which subsequently restores localization of rhodopsin and visual function.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

