Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting
- PMID: 22847373
- PMCID: PMC3426617
- DOI: 10.1097/YCT.0b013e31824f8296
Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting
Abstract
Objectives: Studies now provide strong evidence that the N-methyl-D-aspartate receptor antagonist ketamine possesses rapidly acting antidepressant properties. This study aimed to determine if a low dose of ketamine could be used to expedite and augment the antidepressant effects of electroconvulsive therapy (ECT) treatments in patients experiencing a severe depressive episode.
Materials and methods: Subjects with major depressive disorder or bipolar disorder referred for ECT treatment of a major depressive episode were randomized to receive thiopental alone or thiopental plus ketamine (0.5 mg/kg) for anesthesia before each ECT session. The Hamilton Depression Rating Scale (HDRS) was administered at baseline and at 24 to 72 hours after the first and sixth ECT sessions.
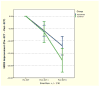
Results: Electroconvulsive therapy exerted a significant antidepressant effect in both groups (F2,24 = 14.35, P < 0.001). However, there was no significant group effect or group-by-time interaction on HDRS scores. In addition, post hoc analyses of the time effect on HDRS showed no significant HDRS reduction after the first ECT session for either group.
Conclusions: The results of this pilot study suggest that ketamine, at a dose of 0.5 mg/kg, given just before ECT, did not enhance the antidepressant effect of ECT. Interestingly, the results further suggest that the coadministration of ketamine with a barbiturate anesthetic and ECT may attenuate the immediate antidepressant effects of the N-methyl-D-aspartate antagonist.
Conflict of interest statement
This work was supported by the State of Connecticut, Department of Mental Health and Addiction Services through its support for the Connecticut Mental Health Center. National Institute of Mental Health (K02 MH076222-04)(GS), and National Institute on Drug Abuse (T32-DA022975)(CGA).
G.S. has received consulting fees from Abbott, AstraZeneca, Avanier Pharmaceuticals, Bristol-Myers Squibb, Evotec, Eli Lilly & Co., Hoffman La-Roche, Johnson & Johnson, Novartis and Novum Pharmaceuticals over the past 24 months. He has also received additional grant support from AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Merck & Co. and Sepracor Inc. over the past 24 months. In addition, he is a co-inventor on a filed patent application by Yale University (PCTWO06108055A1). C.G.A., M.F., B.K. and R.O. report no competing financial interests.
Figures
References
-
- Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. - PubMed
-
- Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. - PubMed
-
- aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

