Islet amyloid polypeptide in pancreatic islets from type 2 diabetic subjects
- PMID: 22847497
- PMCID: PMC3442820
- DOI: 10.4161/isl.20477
Islet amyloid polypeptide in pancreatic islets from type 2 diabetic subjects
Abstract
Aims/hypothesis: Islet amyloid polypeptide (IAPP) is a chief constituent of amyloid deposits in pancreatic islets, characteristic histopathology for type 2 diabetes. The goal of this study was to analyze islet cell composition in diabetic islets for the process of transforming water-soluble IAPP in β-cells to water-insoluble amyloid deposits by Immunocytochemical staining using different dilutions of anti-IAPP antibody. IAPP in β-cell granules may initiate β-cell necrosis through apoptosis to form interstitial amyloid deposits in type 2 diabetic islets.
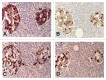
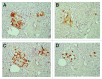
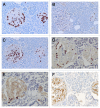
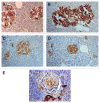
Results: Control islets revealed twice as much β-cells as α-cells whereas 15 of 18 type 2 diabetic cases (83%) revealed α- cells as major cells in larger islets. Diabetic islets consisted of more larger islets with more σ-cells than β-cells, which contribute to hyperglucagonemia. In control islets, percentage of IAPP-positive cells against β-cells was 40-50% whereas percentage for type 2 diabetic islets was about 25%. Amyloid deposits in diabetic islets were not readily immunostained for IAPP using 1: 800 diluted antibody, however, 1: 400 and 1: 200 diluted solutions provided stronger immunostaining in early stages of islet amyloidogenesis after treating the deparaffinized sections with formic acid.
Methods: Using commercially available rabbit antihuman IAPP antibody, immunocytochemical staining was performed on 18 cases of pancreatic tissues from type 2 diabetic subjects by systematically immunostaining for insulin, glucagon, somatostatin (SRIF) and IAPP compared with controls. Sizes of islets were measured by 1 cm scale, mounted in 10X eye piece.
Conclusions/interpretation: α cells were major islet cells in majority of diabetic pancreas (83%) and all diabetic islets contained less IAPP-positive cells than controls, indicating that IAPP deficiency in pancreatic islets is responsible for decreased IAPP in blood. In diabetic islets, water-soluble IAPP disappeared in β-cell granules, which transformed to water-insoluble amyloid deposits. Amyloid deposits were not readily immunostained using IAPP 1: 800 diluted antibody but were stronger immunostained for IAPP in early stages of amyloid deposited islets using less diluted solutions after formic acid treatment. In early islet amyloidogenesis, dying β-cell cytoplasm was adjacently located to fine amyloid fibrils, supporting that IAPP in secretary granules from dying β cells served as nidus for islet β-sheet formation.
Figures

Similar articles
-
Islet amyloid polypeptide in pancreatic islets from type 1 diabetic subjects.Islets. 2011 Jul-Aug;3(4):166-74. doi: 10.4161/isl.3.4.15875. Epub 2011 Jul 1. Islets. 2011. PMID: 21633185 Free PMC article.
-
Immunocytochemical staining for islet amyloid polypeptide in pancreatic endocrine tumors.Islets. 2011 Nov-Dec;3(6):344-51. doi: 10.4161/isl.3.6.17866. Epub 2011 Nov 1. Islets. 2011. PMID: 21983096 Free PMC article.
-
Distribution of pancreatic endocrine cells including IAPP-expressing cells in non-diabetic and type 2 diabetic cases.J Histochem Cytochem. 2007 Feb;55(2):111-8. doi: 10.1369/jhc.6A7024.2006. Epub 2006 Sep 18. J Histochem Cytochem. 2007. PMID: 16982850
-
Islet amyloid polypeptide, islet amyloid, and diabetes mellitus.Physiol Rev. 2011 Jul;91(3):795-826. doi: 10.1152/physrev.00042.2009. Physiol Rev. 2011. PMID: 21742788 Review.
-
Islet amyloid in type 2 (non-insulin-dependent) diabetes.APMIS. 1996 Jan;104(1):12-8. doi: 10.1111/j.1699-0463.1996.tb00680.x. APMIS. 1996. PMID: 8645452 Review.
Cited by
-
Hydroxychloroquine hindering of diabetic isletopathy carries its signature on the inflammatory cytokines.J Mol Histol. 2016 Apr;47(2):183-93. doi: 10.1007/s10735-016-9664-5. Epub 2016 Feb 13. J Mol Histol. 2016. PMID: 26872459
-
New insights into the architecture of the islet of Langerhans: a focused cross-species assessment.Diabetologia. 2015 Oct;58(10):2218-28. doi: 10.1007/s00125-015-3699-0. Epub 2015 Jul 28. Diabetologia. 2015. PMID: 26215305 Review.
-
Coordinated Actions of FXR and LXR in Metabolism: From Pathogenesis to Pharmacological Targets for Type 2 Diabetes.Int J Endocrinol. 2014;2014:751859. doi: 10.1155/2014/751859. Epub 2014 Apr 28. Int J Endocrinol. 2014. PMID: 24872814 Free PMC article. Review.
-
The role of inflammasomes in human diseases and their potential as therapeutic targets.Signal Transduct Target Ther. 2024 Jan 5;9(1):10. doi: 10.1038/s41392-023-01687-y. Signal Transduct Target Ther. 2024. PMID: 38177104 Free PMC article. Review.
-
Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2022 Aug 18;13:976465. doi: 10.3389/fendo.2022.976465. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060972 Free PMC article. Review.
References
-
- Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
