Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis
- PMID: 22847613
- PMCID: PMC3705163
- DOI: 10.1038/onc.2012.334
Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis
Abstract
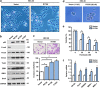
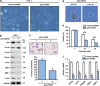
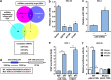
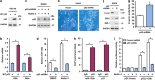
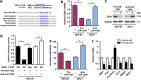
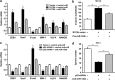
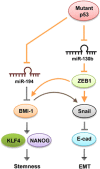
The tumor suppressor gene p53 has been implicated in the regulation of epithelial-mesenchymal transition (EMT) and tumor metastasis by regulating microRNA (miRNA) expression. Here, we report that mutant p53 exerts oncogenic functions and promotes EMT in endometrial cancer (EC) by directly binding to the promoter of miR-130b (a negative regulator of ZEB1) and inhibiting its transcription. We transduced p53 mutants into p53-null EC cells, profiled the miRNA expression by miRNA microarray and identified miR-130b as a potential target of mutant p53. Ectopic expression of p53 mutants repressed the expression of miR-130b and triggered ZEB1-dependent EMT and cancer cell invasion. Loss of an endogenous p53 mutation increased the expression of miR-130b, which resulted in reduced ZEB1 expression and attenuation of the EMT phenotype. Furthermore, re-expression of miR-130b suppressed mutant p53-induced EMT and ZEB1 expression. Importantly, the expression of miR-130 was significantly reduced in EC tissues, and patients with higher expression levels of miR-130b survived longer. These data provide a novel understanding of the roles of p53 gain-of-function mutations in accelerating tumor progression and metastasis through modulation of the miR-130b-ZEB1 axis.
Figures

References
-
- Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

