Inhibition of GSK3 abolishes bacterial-induced periodontal bone loss in mice
- PMID: 22847803
- PMCID: PMC3510296
- DOI: 10.2119/molmed.2012.00180
Inhibition of GSK3 abolishes bacterial-induced periodontal bone loss in mice
Abstract
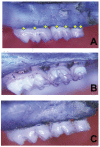
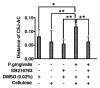
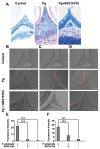
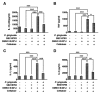
The tissue destruction that characterizes periodontitis is driven by the host response to bacterial pathogens. Inhibition of glycogen synthase kinase 3β (GSK3β) in innate cells leads to suppression of Toll-like receptor (TLR)-initiated proinflammatory cytokines under nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 transcriptional control and promotion of cyclic adenosine monophosphate response element-binding (CREB)-dependent gene activation. Therefore, we hypothesized that the cell permeable GSK3-specific inhibitor, SB216763, would protect against alveolar bone loss induced by the key periodontal pathogen, Porphyromonas gingivalis (P. gingivalis), in a murine model. B6129SF2/J mice either were infected orally with P. gingivalis ATCC 33277; or treated with SB216763 and infected with P. gingivalis; sham infected; or exposed to vehicle only (dimethyl sulfoxide [DMSO]); or to GSK3 inhibitor only (SB216763). Alveolar bone loss and local (neutrophil infiltration and interleukin [IL]-17) and systemic (tumor necrosis factor [TNF], IL-6, Il-1β and IL-12/IL-23 p40) inflammatory indices also were monitored. SB216763 unequivocally abrogated mean P. gingivalis-induced bone resorption, measured at 14 predetermined points on the molars of defleshed maxillae as the distance from the cementoenamel junction to the alveolar bone crest (p < 0.05). The systemic cytokine response, the local neutrophil infiltration and the IL-17 expression were suppressed (p < 0.001). These data confirm the relevance of prior in vitro phenomena and establish GSK3 as a novel, efficacious therapeutic preventing periodontal disease progression in a susceptible host. These findings also may have relevance to other chronic inflammatory diseases and the systemic sequelae associated with periodontal infections.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

