Correlating macrophage morphology and cytokine production resulting from biomaterial contact
- PMID: 22847892
- PMCID: PMC3488130
- DOI: 10.1002/jbm.a.34309
Correlating macrophage morphology and cytokine production resulting from biomaterial contact
Abstract
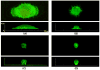
The morphological and inflammatory responses of adherent macrophages are correlated to evaluate the biocompatibility of surfaces. Monocyte-derived macrophage (MDM), THP-1, and THP-1 cells expressing GFP-actin chimeric protein were seeded onto glass, polyurethane (PU), and glass surface modified with quaternary ammonium salt functionalized chitosan (CH-Q) and hyaluronic acid (HA). Using confocal microscopy, the surface area, volume and 3D shape factor of adherent macrophages was quantified. For comparison, functional consequences of cell-surface interactions that activate macrophages and thereby elicit secretion of a proinflammatory cytokine were evaluated. Using an enzyme linked immune sorbent assay, tumor necrosis factor-alpha (TNF-α) was measured. On glass, macrophages exhibited mainly an amoeboid shape, exhibited the largest surface area, volume, and 3D shape factor and produced the most TNF-α. On PU, macrophages displayed mainly a hemispherical shape, exhibited an intermediate volume, surface area and 3D shape factor, and produced moderate TNF-α. In contrast, on CH-Q and HA surfaces, macrophages were spherical, exhibited the smallest volume, surface area, and 3D shape factor, and produced the least TNF-α. These studies begin to validate the use of GFP-actin-modified MDM as a novel tool to correlate cell morphology with inflammatory cell response.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
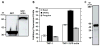

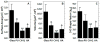
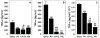
References
-
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. Crit Care Med. 1999;27:887–892. - PubMed
-
- Markowicz P, Wolff M, Djedaini K, Cohen Y, Chastre J, Delclaux C, Merrer J, Herman B, Veber B, Fontaine A, Dreyfuss D. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome - Incidence, prognosis, and risk factors. Am J Resp Crit Care. 2000;161:1942–1948. - PubMed
-
- Martin DC, O’Ryan FS, Indresano AT, Bogdanos P, Wang B, Hui RL, Lo JC. Characteristics of implant failures in patients with a history of oral bisphosphonate therapy. J Oral Maxil Surg. 2010;68:508–514. - PubMed
-
- Baddour LM, Bettmann MA, Bolger AF, Epstein AE, Ferrieri P, Gerber MA, Gewitz MH, Jacobs AK, Levison ME, Newburger JW, Pallasch TJ, Wilson WR, Baltimore RS, Falace DA, Shulman ST, Tani LY, Taubert KA. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015–2031. - PubMed
-
- Bernacca GM, Mackay TG, Wilkinson R, Wheatley DJ. Calcification and fatigue failure in a polyurethane heart valve. Biomaterials. 1995;16:279–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

