Targeting subcellular localization through the polo-box domain: non-ATP competitive inhibitors recapitulate a PLK1 phenotype
- PMID: 22848093
- PMCID: PMC3711794
- DOI: 10.1158/1535-7163.MCT-12-0006-T
Targeting subcellular localization through the polo-box domain: non-ATP competitive inhibitors recapitulate a PLK1 phenotype
Abstract
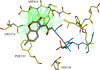
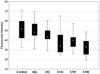
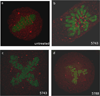
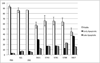
The polo-box domain (PBD) has critical roles in the mitotic functions of polo-like kinase 1 (PLK1). The replacement with partial ligand alternative through computational enrichment (REPLACE) strategy to develop inhibitors of protein-protein interactions has identified alternatives for the N-terminal tripeptide of a Cdc25C substrate. In addition, a peptide structure-activity relationship described key determinants and novel information useful for drug design. Fragment-ligated inhibitory peptides (FLIP) were generated with comparable affinity to peptide PBD inhibitors and possessed antiproliferative phenotypes in cells consistent with the observed decrease in PLK1 centrosomal localization. These FLIPs showed evidence of enhanced PLK1 inhibition in cells relative to peptides and induced monopolar and multipolar spindles, which stands in contrast to previously reported small-molecule PBD inhibitors that display phenotypes only partially representative of PLK1 knockdown. Progress obtained applying REPLACE validates this approach for identifying fragment alternatives for determinants of the Cdc25C-binding motif and extends its applicability of the strategy for discovering protein-protein interaction inhibitors. In addition, the described PBD inhibitors retain high specificity for PLK1 over PLK3 and therefore show promise as isotype selective, non-ATP competitive kinase inhibitors that provide new impetus for the development of PLK1-selective antitumor therapeutics.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to disclose.
Figures

Similar articles
-
Identification of a novel Polo-like kinase 1 inhibitor that specifically blocks the functions of Polo-Box domain.Oncotarget. 2017 Jan 3;8(1):1234-1246. doi: 10.18632/oncotarget.13603. Oncotarget. 2017. PMID: 27902479 Free PMC article.
-
Peptidomimetic Polo-Box-Targeted Inhibitors that Engage PLK1 in Tumor Cells and Are Selective against the PLK3 Tumor Suppressor.ChemMedChem. 2020 Jun 17;15(12):1058-1066. doi: 10.1002/cmdc.202000137. Epub 2020 Apr 28. ChemMedChem. 2020. PMID: 32232973 Free PMC article.
-
Inhibitors of the Polo-Box Domain of Polo-Like Kinase 1.Chembiochem. 2016 Apr 15;17(8):650-6. doi: 10.1002/cbic.201500580. Epub 2016 Jan 29. Chembiochem. 2016. PMID: 26662918 Review.
-
Enhancing polo-like kinase 1 selectivity of polo-box domain-binding peptides.Bioorg Med Chem. 2017 Oct 1;25(19):5041-5049. doi: 10.1016/j.bmc.2017.02.063. Epub 2017 Feb 28. Bioorg Med Chem. 2017. PMID: 28285924 Free PMC article.
-
Molecular and enzoinformatics perspectives of targeting Polo-like kinase 1 in cancer therapy.Semin Cancer Biol. 2019 Jun;56:47-55. doi: 10.1016/j.semcancer.2017.11.004. Epub 2017 Nov 6. Semin Cancer Biol. 2019. PMID: 29122685 Review.
Cited by
-
Exploring the binding nature of pyrrolidine pocket-dependent interactions in the polo-box domain of polo-like kinase 1.PLoS One. 2013 Nov 6;8(11):e80043. doi: 10.1371/journal.pone.0080043. eCollection 2013. PLoS One. 2013. PMID: 24223211 Free PMC article.
-
Identifying novel SMYD3 interactors on the trail of cancer hallmarks.Comput Struct Biotechnol J. 2022 Apr 11;20:1860-1875. doi: 10.1016/j.csbj.2022.03.037. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35495117 Free PMC article.
-
Design and synthesis of a cell-permeable, drug-like small molecule inhibitor targeting the polo-box domain of polo-like kinase 1.PLoS One. 2014 Sep 11;9(9):e107432. doi: 10.1371/journal.pone.0107432. eCollection 2014. PLoS One. 2014. PMID: 25211362 Free PMC article.
-
Identification of a novel Polo-like kinase 1 inhibitor that specifically blocks the functions of Polo-Box domain.Oncotarget. 2017 Jan 3;8(1):1234-1246. doi: 10.18632/oncotarget.13603. Oncotarget. 2017. PMID: 27902479 Free PMC article.
-
Structure-activity and mechanistic studies of non-peptidic inhibitors of the PLK1 polo box domain identified through REPLACE.Eur J Med Chem. 2022 Jan 5;227:113926. doi: 10.1016/j.ejmech.2021.113926. Epub 2021 Oct 21. Eur J Med Chem. 2022. PMID: 34735919 Free PMC article.
References
-
- Xie S, Xie B, Lee MY, Dai W. Regulation of cell cycle checkpoints by polo-like kinases. Oncogene. 2005;24:277–286. - PubMed
-
- McInnes C, Wyatt MD. PLK1 as an oncology target: current status and future potential. Drug Discov Today. 2011;16:619–625. - PubMed
-
- Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543–549. - PubMed
-
- Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. International Journal of Oncology. 1999;15:687–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

