The effect of erythropoietin on normal and neoplastic cells
- PMID: 22848149
- PMCID: PMC3402043
- DOI: 10.2147/BTT.S32281
The effect of erythropoietin on normal and neoplastic cells
Abstract
Erythropoietin (Epo) is an essential hormone that binds and activates the Epo receptor (EpoR) resident on the surface of erythroid progenitor cells, thereby promoting erythropoiesis. Recombinant human erythropoietin has been used successfully for over 20 years to treat anemia in millions of patients. In addition to erythropoiesis, Epo has also been reported to have other effects, such as tissue protection and promotion of tumor cell growth or survival. This became of significant concern in 2003, when some clinical trials in cancer patients reported increased tumor progression and worse survival outcomes in patients treated with erythropoiesis-stimulating agents (ESAs). One of the potential mechanisms proffered to explain the observed safety issues was that functional EpoR was expressed in tumors and/or endothelial cells, and that ESAs directly stimulated tumor growth and/or antagonized tumor ablative therapies. Since then, numerous groups have performed further research evaluating this potential mechanism with conflicting data and conclusions. Here, we review the biology of endogenous Epo and EpoR expression and function in erythropoiesis, and evaluate the evidence pertaining to the expression of EpoR on normal nonhematopoietic and tumor cells.
Keywords: anemia; angiogenesis; erythropoietin; erythropoietin receptor; tumor.
Figures
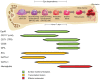

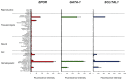


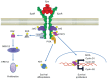
References
-
- Krantz SB. Erythropoietin. Blood. 1991;77(3):419–434. - PubMed
-
- Bohlius J, Tonia T, Schwarzer G. Twist and shout: one decade of metaanalyses of erythropoiesis-stimulating agents in cancer patients. Acta Haematol. 2010;125(1–2):55–67. - PubMed
-
- Papayannopoulou T, Abkowitz J, D’Andrea A. Biology of erythropoiesis, erythroid maturation and differentiation. In: Hoffman R, Benz EJ, Shattil SJ, et al., editors. Hematology Basic Principals and Practice. 3rd ed. Philadelphia: Churchill Livingston; 2000. pp. 202–219.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

