Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus
- PMID: 22848375
- PMCID: PMC3407224
- DOI: 10.1371/journal.pone.0040401
Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus
Abstract
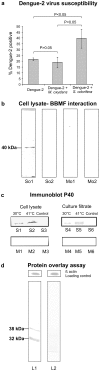
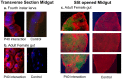
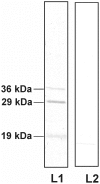
Mosquito midgut plays a crucial role in its vector susceptibility and pathogen interaction. Identification of the sustainable microflora of the midgut environment can therefore help in evaluating its contribution in mosquito-pathogen interaction and in turn vector competence. To understand the bacterial diversity in the midgut of Aedes aegypti mosquitoes, we conducted a screening study of the gut microbes of these mosquitoes which were either collected from fields or reared in the laboratory "culture-dependent" approach. This work demonstrated that the microbial flora of larvae and adult Ae. aegypti midgut is complex and is dominated by gram negative proteobacteria. Serratia odorifera was found to be stably associated in the midguts of field collected and laboratory reared larvae and adult females. The potential influence of this sustainable gut microbe on DENV-2 susceptibility of this vector was evaluated by co-feeding S. odorifera with DENV-2 to adult Ae. aegypti females (free of gut flora). The observations revealed that the viral susceptibility of these Aedes females enhanced significantly as compared to solely dengue-2 fed and another gut inhabitant, Microbacterium oxydans co-fed females. Based on the results of this study we proposed that the enhancement in the DENV-2 susceptibility of Ae. aegypti females was due to blocking of prohibitin molecule present on the midgut surface of these females by the polypeptide of gut inhabitant S. odorifera.
Conflict of interest statement
Figures
References
-
- Dillon RJ, Vennard CT, Buckling A, Charnley AK (2005) Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett 8: 1291–1298.
-
- Dong Y, Manfredini F, Dimopoulos G (2009) Implication of the Mosquito Midgut Microbiota in the defence against Malaria Parasites. PLoS Pathog 5: e1000423 doi:10.1371/journal.ppat.1000423. - DOI - PMC - PubMed
-
- Jadin J, Vincke IH, Dunjic A, Delville JP, Wery M, et al. (1966) Role of Pseudomonas in the sporogenesis of the hematozoon of malaria in the mosquito. Bull Soc Pathol Exot Filiales 59: 514–525. - PubMed
-
- Seitz HM, Maier WA, Rottok M, Becker-Feldmann H (1987) Concomitant infections of Anopheles stephensi with Plasmodium berghei and Serratia marcescens: additive detrimental effects. Zentralbl Bakteriol Hyg 266: 155–166. - PubMed
-
- Pumpuni CB, DeMaio J, Kent M, Davis JR, Beier JC (1996) Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg 54: 214–218. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

