Upregulation of the coagulation factor VII gene during glucose deprivation is mediated by activating transcription factor 4
- PMID: 22848420
- PMCID: PMC3407153
- DOI: 10.1371/journal.pone.0040994
Upregulation of the coagulation factor VII gene during glucose deprivation is mediated by activating transcription factor 4
Abstract
Background: Constitutive production of blood coagulation proteins by hepatocytes is necessary for hemostasis. Stressful conditions trigger adaptive cellular responses and delay processing of most proteins, potentially affecting plasma levels of proteins secreted exclusively by hepatocytes. We examined the effect of glucose deprivation on expression of coagulation proteins by the human hepatoma cell line, HepG2.
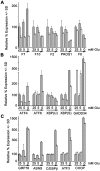
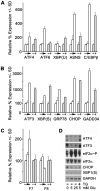
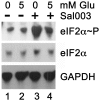
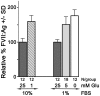
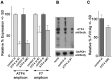
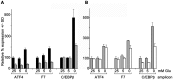
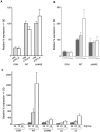
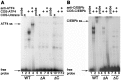
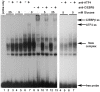
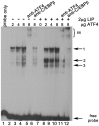
Methodology/principal findings: Expression of coagulation factor VII, which is required for initiation of blood coagulation, was elevated by glucose deprivation, while expression of other coagulation proteins decreased. Realtime PCR and ELISA demonstrated that the relative percentage expression +/- SD of steady-state F7 mRNA and secreted factor VII antigen were significantly increased (from 100+/-15% to 188+/-27% and 100+/-8.8% to 176.3+/-17.3% respectively, p<0.001) at 24 hr of treatment. The integrated stress response was induced, as indicated by upregulation of transcription factor ATF4 and of additional stress-responsive genes. Small interfering RNAs directed against ATF4 potently reduced basal F7 expression, and prevented F7 upregulation by glucose deprivation. The response of the endogenous F7 gene was replicated in reporter gene assays, which further indicated that ATF4 effects were mediated via interaction with an amino acid response element in the F7 promoter.
Conclusions/significance: Our data indicated that glucose deprivation enhanced F7 expression in a mechanism reliant on prior ATF4 upregulation primarily due to increased transcription from the ATF4 gene. Of five coagulation protein genes examined, only F7 was upregulated, suggesting that its functions may be important in a systemic response to glucose deprivation stress.
Conflict of interest statement
Figures
References
-
- Fair DS, Plow EF (1983) Synthesis and secretion of the fibrinolytic components, including alpha 2-antiplasmin, by a human hepatoma cell line. J Lab Clin Med 101: 372–384. - PubMed
-
- Monroe DM, Hoffman M (2006) What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol 26: 41–48. - PubMed
-
- Mackman N (2006) Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis 36: 104–107. - PubMed
-
- Dahlback B, Villoutreix BO (2005) Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol 25: 1311–1320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

