Subthalamic nucleus electrical stimulation modulates calcium activity of nigral astrocytes
- PMID: 22848608
- PMCID: PMC3407058
- DOI: 10.1371/journal.pone.0041793
Subthalamic nucleus electrical stimulation modulates calcium activity of nigral astrocytes
Abstract
Background: The substantia nigra pars reticulata (SNr) is a major output nucleus of the basal ganglia, delivering inhibitory efferents to the relay nuclei of the thalamus. Pathological hyperactivity of SNr neurons is known to be responsible for some motor disorders e.g. in Parkinson's disease. One way to restore this pathological activity is to electrically stimulate one of the SNr input, the excitatory subthalamic nucleus (STN), which has emerged as an effective treatment for parkinsonian patients. The neuronal network and signal processing of the basal ganglia are well known but, paradoxically, the role of astrocytes in the regulation of SNr activity has never been studied.
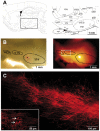
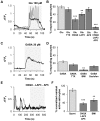
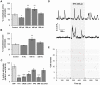
Principal findings: In this work, we developed a rat brain slice model to study the influence of spontaneous and induced excitability of afferent nuclei on SNr astrocytes calcium activity. Astrocytes represent the main cellular population in the SNr and display spontaneous calcium activities in basal conditions. Half of this activity is autonomous (i.e. independent of synaptic activity) while the other half is dependent on spontaneous glutamate and GABA release, probably controlled by the pace-maker activity of the pallido-nigral and subthalamo-nigral loops. Modification of the activity of the loops by STN electrical stimulation disrupted this astrocytic calcium excitability through an increase of glutamate and GABA releases. Astrocytic AMPA, mGlu and GABA(A) receptors were involved in this effect.
Significance: Astrocytes are now viewed as active components of neural networks but their role depends on the brain structure concerned. In the SNr, evoked activity prevails and autonomous calcium activity is lower than in the cortex or hippocampus. Our data therefore reflect a specific role of SNr astrocytes in sensing the STN-GPe-SNr loops activity and suggest that SNr astrocytes could potentially feedback on SNr neuronal activity. These findings have major implications given the position of SNr in the basal ganglia network.
Conflict of interest statement
Figures


References
-
- Hikosaka O. GABAergic output of the basal ganglia. Prog Brain Res. 2007;160:209–226. - PubMed
-
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. - PubMed
-
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. - PubMed
-
- Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. - PubMed
-
- Kitai ST, Deniau JM. Cortical inputs to the subthalamus: intracellular analysis. Brain Res. 1981;214:411–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

