In vitro expanded stem cells from the developing retina fail to generate photoreceptors but differentiate into myelinating oligodendrocytes
- PMID: 22848612
- PMCID: PMC3405018
- DOI: 10.1371/journal.pone.0041798
In vitro expanded stem cells from the developing retina fail to generate photoreceptors but differentiate into myelinating oligodendrocytes
Abstract
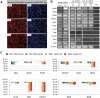
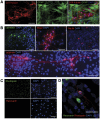
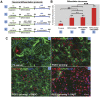
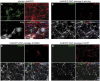
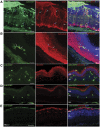
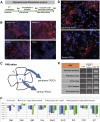
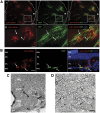
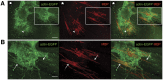
Cell transplantation to treat retinal degenerative diseases represents an option for the replacement of lost photoreceptor cells. In vitro expandable cells isolated from the developing mammalian retina have been suggested as a potential source for the generation of high numbers of donor photoreceptors. In this study we used standardized culture conditions based on the presence of the mitogens FGF-2 and EGF to generate high numbers of cells in vitro from the developing mouse retina. These presumptive 'retinal stem cells' ('RSCs') can be propagated as monolayer cultures over multiple passages, express markers of undifferentiated neural cells, and generate neuronal and glial cell types upon withdrawal of mitogens in vitro or following transplantation into the adult mouse retina. The proportion of neuronal differentiation can be significantly increased by stepwise removal of mitogens and inhibition of the notch signaling pathway. However, 'RSCs', by contrast to their primary counterparts in vivo, i.e. retinal progenitor cells, loose the expression of retina-specific progenitor markers like Rax and Chx10 after passaging and fail to differentiate into photoreceptors both in vitro or after intraretinal transplantation. Notably, 'RSCs' can be induced to differentiate into myelinating oligodendrocytes, a cell type not generated by primary retinal progenitor cells. Based on these findings we conclude that 'RSCs' expanded in high concentrations of FGF-2 and EGF loose their retinal identity and acquire features of in vitro expandable neural stem-like cells making them an inappropriate cell source for strategies aimed at replacing photoreceptor cells in the degenerated retina.
Conflict of interest statement
Figures
Similar articles
-
Isolation of retinal progenitor and stem cells from the porcine eye.Mol Vis. 2007 Jun 29;13:1045-57. Mol Vis. 2007. PMID: 17653049 Free PMC article.
-
Fibroblast growth factor and epidermal growth factor differently affect differentiation of murine retinal stem cells in vitro.Mol Vis. 2007 Oct 2;13:1842-50. Mol Vis. 2007. PMID: 17960120
-
Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate.Invest Ophthalmol Vis Sci. 2007 Jan;48(1):446-54. doi: 10.1167/iovs.06-0190. Invest Ophthalmol Vis Sci. 2007. PMID: 17197566 Free PMC article.
-
An essential role for Rax in retina and neuroendocrine system development.Dev Growth Differ. 2012 Apr;54(3):341-8. doi: 10.1111/j.1440-169X.2012.01337.x. Dev Growth Differ. 2012. PMID: 22524605 Review.
-
Stem cell therapy and the retina.Eye (Lond). 2007 Oct;21(10):1352-9. doi: 10.1038/sj.eye.6702842. Eye (Lond). 2007. PMID: 17914439 Review.
Cited by
-
Immunocytochemical Profiling of Cultured Mouse Primary Retinal Cells.J Histochem Cytochem. 2017 Apr;65(4):223-239. doi: 10.1369/0022155416689675. Epub 2017 Feb 2. J Histochem Cytochem. 2017. PMID: 28151698 Free PMC article.
-
VSX2 and ASCL1 Are Indicators of Neurogenic Competence in Human Retinal Progenitor Cultures.PLoS One. 2015 Aug 20;10(8):e0135830. doi: 10.1371/journal.pone.0135830. eCollection 2015. PLoS One. 2015. PMID: 26292211 Free PMC article.
-
Cellular regeneration strategies for macular degeneration: past, present and future.Eye (Lond). 2018 May;32(5):946-971. doi: 10.1038/s41433-018-0061-z. Epub 2018 Mar 5. Eye (Lond). 2018. PMID: 29503449 Free PMC article. Review.
-
Generation of Human Corneal Endothelial Cells via In Vitro Ocular Lineage Restriction of Pluripotent Stem Cells.Invest Ophthalmol Vis Sci. 2016 Dec 1;57(15):6878-6884. doi: 10.1167/iovs.16-20024. Invest Ophthalmol Vis Sci. 2016. PMID: 28002562 Free PMC article.
-
What can we learn about stroke from retinal ischemia models?Acta Pharmacol Sin. 2013 Jan;34(1):91-103. doi: 10.1038/aps.2012.165. Epub 2012 Dec 3. Acta Pharmacol Sin. 2013. PMID: 23202803 Free PMC article. Review.
References
-
- Delyfer MN, Leveillard T, Mohand-Said S, Hicks D, Picaud S, et al. Inherited retinal degenerations: therapeutic prospects. Biology of the cell/under the auspices of the European Cell Biology Organization. 2004;96:261–269. - PubMed
-
- Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Moser A, et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Archives of ophthalmology. 2004;122:1297–1305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

