Expressional changes in cerebrovascular receptors after experimental transient forebrain ischemia
- PMID: 22848635
- PMCID: PMC3407123
- DOI: 10.1371/journal.pone.0041852
Expressional changes in cerebrovascular receptors after experimental transient forebrain ischemia
Abstract
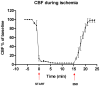
Background: Global ischemic stroke is one of the most prominent consequences of cardiac arrest, since the diminished blood flow to the brain results in cell damage and sometimes permanently impaired neurological function. The post-arrest period is often characterised by cerebral hypoperfusion due to subacute hemodynamic disturbances, the pathophysiology of which are poorly understood. In two other types of stroke, focal ischemic stroke and subarachnoid hemorrhage, it has earlier been demonstrated that the expression of certain vasoconstrictor receptors is increased in cerebral arteries several days after the insult, a phenomenon that leads to increased contraction of cerebral arteries, reduced perfusion of the affected area and worsened ischemic damage. Based on these findings, the aim of the present study was to investigate if transient global cerebral ischemia is associated with upregulation of vasoconstrictive endothelin and 5-hydroxytryptamine receptors in cerebral arteries. Experimental transient forebrain ischemia of varying durations was induced in male wistar rats, followed by reperfusion for 48 hours. Neurological function was assessed daily by three different tests and cerebrovascular expression and contractile function of endothelin and 5-hydroxytryptamine receptors were evaluated by wire myography, immunohistochemistry and western blotting.
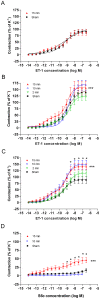
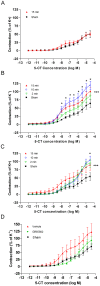
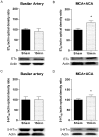
Results: Transient forebrain ischemia induced neurological deficits as well as functional upregulation of vasoconstrictive ET(B) and 5-HT(1B) receptors in cerebral arteries supplying mid- and forebrain regions. No receptor upregulation was seen in arteries supplying the hindbrain. Immunohistochemical stainings and western blotting demonstrated expressional upregulation of these receptor subtypes in the mid- and forebrain arteries and confirmed that the receptors were located in the smooth muscle layer of the cerebral arteries.
Conclusions: This study reveals a new pathophysiological aspect of global ischemic stroke, namely expressional upregulation of vasoconstrictor receptors in cerebral arteries two days after the insult, which might contribute to cerebral hypoperfusion and delayed neuronal damage after cardiac arrest.
Conflict of interest statement
Figures
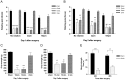
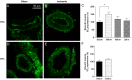
 SEM in percentages of the mean staining intensity in control-operated (sham) animals. Significant differences between sham-operated and 15 minutes ischemia rats were determined using student´s t-test. *p< 0.05.
SEM in percentages of the mean staining intensity in control-operated (sham) animals. Significant differences between sham-operated and 15 minutes ischemia rats were determined using student´s t-test. *p< 0.05.

References
-
- Hossmann KA (2008) Cerebral ischemia: models, methods and outcomes. Neuropharmacology 55: 257–270. S0028–3908(07)00391–7 [pii];10.1016/j.neuropharm.2007.12.004 [doi]. - DOI - PubMed
-
- Schneider A, Bottiger BW, Popp E (2009) Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg 108: 971–979. 108/3/971 [pii];10.1213/ane.0b013e318193ca99 [doi]. - DOI - PubMed
-
- Harukuni I, Bhardwaj A (2006) Mechanisms of brain injury after global cerebral ischemia. Neurol Clin 24: 1–21. S0733–8619(05)00084–8 [pii];10.1016/j.ncl.2005.10.004 [doi]. - DOI - PubMed
-
- Bass E (1985) Cardiopulmonary arrest. Pathophysiology and neurologic complications. Ann Intern Med 103: 920–927. - PubMed
-
- Richmond TS (1997) Cerebral resuscitation after global brain ischemia: linking research to practice. AACN Clin Issues 8: 171–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

