Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death
- PMID: 22848721
- PMCID: PMC3405052
- DOI: 10.1371/journal.pone.0042107
Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death
Abstract
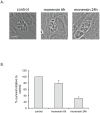
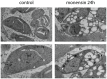
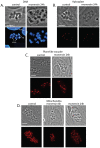
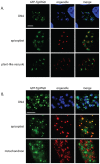
Understanding the mechanisms by which anti-parasitic drugs alter the physiology and ultimately kill is an important area of investigation. Development of novel parasitic drugs, as well as the continued utilization of existing drugs in the face of resistant parasite populations, requires such knowledge. Here we show that the anti-coccidial drug monensin kills Toxoplasma gondii by inducing autophagy in the parasites, a novel mechanism of cell death in response to an antimicrobial drug. Monensin treatment results autophagy, as shown by translocation of ATG8 to autophagosomes, as well as causing marked morphological changes in the parasites' mitochondria. Use of the autophagy inhibitor 3-methyladenine blocks autophagy and mitochondrial alterations, and enhances parasite survival, in monensin-exposed parasites, although it does not block other monensin-induced effects on the parasites, such as late S-phase cell cycle arrest. Monensin does not induce autophagy in a parasite strain deficient in the mitochondrial DNA repair enzyme TgMSH-1 an enzyme that mediates monensin-induced late S-phase arrest. TgMSH-1 therefore either mediates cell cycle arrest and autophagy independently, or autophagy occurs downstream of cell cycle arrest in a manner analogous to apoptosis of cells arrested in G(2) of the cell cycle. Overall, our results point to autophagy as a potentially important mode of cell death of protozoan parasites in response to antimicrobial drugs and indicate that disruption of the autophagy pathway could result in drug resistance.
Conflict of interest statement
Figures

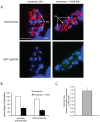

References
-
- Muller J, Hemphill A (2011) Drug target identification in intracellular and extracellular protozoan parasites. Curr Top Med Chem 11: 2029–2038. - PubMed
-
- Haberkorn A (1996) Chemotherapy of human and animal coccidioses: state and perspectives. Parasitol Res 82: 193–199. - PubMed
-
- Mead JR (2002) Cryptosporidiosis and the challenges of chemotherapy. Drug Resist Updat 5: 47–57. - PubMed
-
- Petersen I, Eastman R, Lanzer M (2011) Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett 585: 1551–1562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

