The cellular immune response of the pea aphid to foreign intrusion and symbiotic challenge
- PMID: 22848726
- PMCID: PMC3407134
- DOI: 10.1371/journal.pone.0042114
The cellular immune response of the pea aphid to foreign intrusion and symbiotic challenge
Abstract
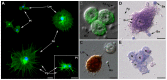
Recent studies suggest that the pea aphid (Acyrthosiphon pisum) has low immune defenses. However, its immune components are largely undescribed, and notably, extensive characterization of circulating cells has been missing. Here, we report characterization of five cell categories in hemolymph of adults of the LL01 pea aphid clone, devoid of secondary symbionts (SS): prohemocytes, plasmatocytes, granulocytes, spherulocytes and wax cells. Circulating lipid-filed wax cells are rare; they otherwise localize at the basis of the cornicles. Spherulocytes, that are likely sub-cuticular sessile cells, are involved in the coagulation process. Prohemocytes have features of precursor cells. Plasmatocytes and granulocytes, the only adherent cells, can form a layer in vivo around inserted foreign objects and phagocytize latex beads or Escherichia coli bacteria injected into aphid hemolymph. Using digital image analysis, we estimated that the hemolymph from one LL01 aphid contains about 600 adherent cells, 35% being granulocytes. Among aphid YR2 lines differing only in their SS content, similar results to LL01 were observed for YR2-Amp (without SS) and YR2-Ss (with Serratia symbiotica), while YR2-Hd (with Hamiltonella defensa) and YR2(Ri) (with Regiella insecticola) had strikingly lower adherent hemocyte numbers and granulocyte proportions. The effect of the presence of SS on A. pisum cellular immunity is thus symbiont-dependent. Interestingly, Buchnera aphidicola (the aphid primary symbiont) and all SS, whether naturally present, released during hemolymph collection, or artificially injected, were internalized by adherent hemocytes. Inside hemocytes, SS were observed in phagocytic vesicles, most often in phagolysosomes. Our results thus raise the question whether aphid symbionts in hemolymph are taken up and destroyed by hemocytes, or actively promote their own internalization, for instance as a way of being transmitted to the next generation. Altogether, we demonstrate here a strong interaction between aphid symbionts and immune cells, depending upon the symbiont, highlighting the link between immunity and symbiosis.
Conflict of interest statement
Figures








References
-
- Broderick NA, Welchman DP, Lemaitre B (2009) Recognition and response to microbial infection in Drosophila. In: Insect Infection and Immunity. Rolff J, Reynolds S, editors. Oxford University Press, USA 13–33.
-
- Cerenius L, Kawabata S-I, Lee BL, Nonaka M, Söderhäll K (2010) Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem Sci 35: 575–583. - PubMed
-
- Nappi A, Poirié M, Carton Y (2009) The Role of melanization and cytotoxic by-products in the cellular immune response of Drosophila against parasitic wasps. Adv Parasitol 70: 99–121. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

