Gemcitabine Plus Carboplatin in Patients with Advanced Hepatocellular Carcinoma: Results of a Phase II Study
- PMID: 22848843
- PMCID: PMC3405685
- DOI: 10.5402/2012/420931
Gemcitabine Plus Carboplatin in Patients with Advanced Hepatocellular Carcinoma: Results of a Phase II Study
Abstract
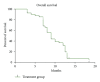
Objectives. Assessment of gemcitabine/carboplatin combination in patients with advanced-stage hepatocellular carcinoma (HCC) in a phase II trial for safety and efficacy. Methods. Forty patients with previously untreated advanced-stage HCC were prospectively enrolled and subjected to gemcitabine/carboplatin regimen which consisted of gemcitabine 1000 mg/m(2) on days 1 and 8, and carboplatin AUC 6 on day 1. The treatment was repeated every 3 weeks until disease progression or limiting toxicity. Results. Forty patients were assessable for efficacy and toxicity. In all, 276 treatment cycles were administered. No toxic deaths occurred. Hematological grade 3-4 toxicity consisted of thrombocytopenia (27% of patients) and neutropenia (24%), including 2 febrile neutropenia and anemia (9%). Grade 3 carboplatin-induced neurotoxicity was observed in 3 (9%) patients. ORR was 23% (95% CI, 0.10-0.29) with 9 partial responses and disease stabilization was observed in 46% (95% CI, 0.22-0.42) of patients, giving a disease control rate of 69%. Median progression-free and overall survival times were, respectively, 5 months (95% CI: 3-8 months) and 8 months (95% CI: 6-18 months). Conclusion. The gemcitabine/carboplatin regimen seems to be effective, well tolerated, and active in advanced HCC.
Figures
References
-
- Anwar WA, Khaled HM, Amra HA, El-Nezami H, Loffredo CA. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: possibilities for prevention. Mutation Research. 2008;659(1-2):176–184. - PubMed
-
- Jelic S, Sotiropoulos GC. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21(supplement 5):v59–v64. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New England Journal of Medicine. 2008;359(4):378–390. - PubMed
LinkOut - more resources
Full Text Sources