Transport of biogenic amine neurotransmitters at the mouse blood-retina and blood-brain barriers by uptake1 and uptake2
- PMID: 22850405
- PMCID: PMC3493996
- DOI: 10.1038/jcbfm.2012.109
Transport of biogenic amine neurotransmitters at the mouse blood-retina and blood-brain barriers by uptake1 and uptake2
Abstract
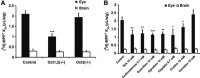
Uptake1 and uptake2 transporters are involved in the extracellular clearance of biogenic amine neurotransmitters at synaptic clefts. We looked for them at the blood-brain barrier (BBB) and blood-retina barriers (BRB), where they could be involved in regulating the neurotransmitter concentration and modulate/terminate receptor-mediated effects within the neurovascular unit (NVU). Uptake2 (Oct1-3/Slc22a1-3, Pmat/Slc29a4) and Mate1/Slc47a1 transporters are also involved in the transport of xenobiotics. We used in situ carotid perfusion of prototypic substrates like [(3)H]-1-methyl-4-phenylpyridinium ([(3)H]-MPP(+)), [(3)H]-histamine, [(3)H]-serotonin, and [(3)H]-dopamine, changes in ionic composition and genetic deletion of Oct1-3 carriers to detect uptake1 and uptake2 at the BBB and BRB. We showed that uptake1 and uptake2 are involved in the transport of [(3)H]-dopamine and [(3)H]-MPP(+) at the blood luminal BRB, but not at the BBB. These functional studies, together with quantitative RT-PCR and confocal imaging, suggest that the mouse BBB lacks uptake1 (Net/Slc6a2, Dat/Slc6a3, Sert/Slc6a4), uptake2, and Mate1 on both the luminal and abluminal sides. However, we found evidence for functional Net and Oct1 transporters at the luminal BRB. These heterogeneous transport properties of the brain and retina NVUs suggest that the BBB helps protect the brain against biogenic amine neurotransmitters in the plasma while the BRB has more of a metabolic/endocrine role.
Figures





References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. - PubMed
-
- Brust P, Friedrich A, Krizbai IA, Bergmann R, Roux F, Ganapathy V, Johannsen B. Functional expression of the serotonin transporter in immortalized rat brain microvessel endothelial cells. J Neurochem. 2000;74:1241–1248. - PubMed
-
- Catravas JD, Gillis CN. Single-pass removal of [14C]-5-hydroxytryptamine and [3H]norepinephrine by rabbit lung, in vivo: kinetics and sites of removal. J Pharmacol Exp Ther. 1983;224:28–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

