Unnatural base pair systems toward the expansion of the genetic alphabet in the central dogma
- PMID: 22850726
- PMCID: PMC3422687
- DOI: 10.2183/pjab.88.345
Unnatural base pair systems toward the expansion of the genetic alphabet in the central dogma
Abstract
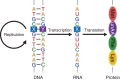
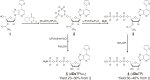
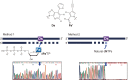
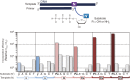
Toward the expansion of the genetic alphabet of DNA, several artificial third base pairs (unnatural base pairs) have been created. Synthetic DNAs containing the unnatural base pairs can be amplified faithfully by PCR, along with the natural A-T and G-C pairs, and transcribed into RNA. The unnatural base pair systems now have high potential to open the door to next generation biotechnology. The creation of unnatural base pairs is a consequence of repeating "proof of concept" experiments. In the process, initially designed base pairs were modified to address their weak points. Some of them were artificially evolved to ones with higher efficiency and selectivity in polymerase reactions, while others were eliminated from the analysis. Here, we describe the process of unnatural base pair development, as well as the tests of their applications.
Figures










References
-
- Watson J.D., Crick F.H. (1953) Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171, 737–738 - PubMed
-
- Nirenberg M., Leder P. (1964) RNA codewords and protein synthesis. The effect of trinucleotides upon the binding of sRNA to ribosomes. Science 145, 1399–1407 - PubMed
-
- Nishimura S., Jones D.S., Khorana H.G. (1965) Studies on polynucleotides. 48. The in vitro synthesis of a co-polypeptide containing two amino acids in alternating sequence dependent upon a DNA-like polymer containing two nucleotides in alternating sequence. J. Mol. Biol. 13, 302–324 - PubMed
-
- Rich, A. (1962) Problems of evolution and biochemical information transfer. In Horizons in Biochemistry (eds. Kasha, M. and Pullman, B.). Academic Press, New York, pp. 103–126.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

