Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity
- PMID: 22850879
- PMCID: PMC3428085
- DOI: 10.1172/JCI62182
Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity
Abstract
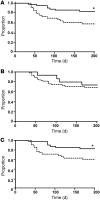
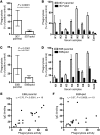
Plasmodium falciparum is the major cause of malaria globally and is transmitted by mosquitoes. During parasitic development, P. falciparum-infected erythrocytes (P. falciparum-IEs) express multiple polymorphic proteins known as variant surface antigens (VSAs), including the P. falciparum erythrocyte membrane protein 1 (PfEMP1). VSA-specific antibodies are associated with protection from symptomatic and severe malaria. However, the importance of the different VSA targets of immunity to malaria remains unclear, which has impeded an understanding of malaria immunity and vaccine development. In this study, we developed assays using transgenic P. falciparum with modified PfEMP1 expression to quantify serum antibodies to VSAs among individuals exposed to malaria. We found that the majority of the human antibody response to the IE targets PfEMP1. Furthermore, our longitudinal studies showed that individuals with PfEMP1-specific antibodies had a significantly reduced risk of developing symptomatic malaria, whereas antibodies to other surface antigens were not associated with protective immunity. Using assays that measure antibody-mediated phagocytosis of IEs, an important mechanism in parasite clearance, we identified PfEMP1 as the major target of these functional antibodies. Taken together, these data demonstrate that PfEMP1 is a key target of humoral immunity. These findings advance our understanding of the targets and mediators of human immunity to malaria and have major implications for malaria vaccine development.
Figures
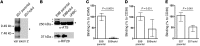
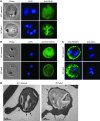
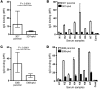
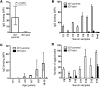
References
-
- World Health Organization . Global Malaria Programme. World Malaria Report 2010. Geneva, Switzerland: World Health Organization; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

