Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens
- PMID: 22850884
- PMCID: PMC3408734
- DOI: 10.1172/JCI60325
Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens
Abstract
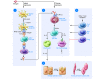
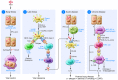
Research on the pathogenesis of asthma has traditionally concentrated on environmental stimuli, genetic susceptibilities, adaptive immune responses, and end-organ alterations (particularly in airway mucous cells and smooth muscle) as critical steps leading to disease. The focus of this cascade has been the response to allergic stimuli. An alternative scheme suggests that respiratory viruses and the consequent response of the innate immune system also drives the development of asthma as well as related inflammatory diseases. This conceptual shift raises the possibility that sentinel cells such as airway epithelial cells, DCs, NKT cells, innate lymphoid cells, and macrophages also represent critical components of asthma pathogenesis as well as new targets for therapeutic discovery. A particular challenge will be to understand and balance the innate as well as the adaptive immune responses to defend the host against acute infection as well as chronic inflammatory disease.
Figures
References
-
- Mosmann TG, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. doi: 10.1146/annurev.iy.07.040189.001045. doi: 10.1146/annurev.iy.07.040189.001045. - DOI - DOI - DOI - PubMed

