Extracellular HSP27 acts as a signaling molecule to activate NF-κB in macrophages
- PMID: 22851137
- PMCID: PMC3508120
- DOI: 10.1007/s12192-012-0356-0
Extracellular HSP27 acts as a signaling molecule to activate NF-κB in macrophages
Abstract
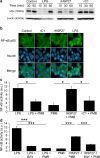
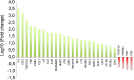
Heat shock protein 27 (HSP27) shows attenuated expression in human coronary arteries as the extent of atherosclerosis progresses. In mice, overexpression of HSP27 reduces atherogenesis, yet the precise mechanism(s) are incompletely understood. Inflammation plays a central role in atherogenesis, and of particular interest is the balance of pro- and anti-inflammatory factors produced by macrophages. As nuclear factor-kappa B (NF-κB) is a key immune signaling modulator in atherogenesis, and macrophages are known to secrete HSP27, we sought to determine if recombinant HSP27 (rHSP27) alters NF-κB signaling in macrophages. Treatment of THP-1 macrophages with rHSP27 resulted in the degradation of an inhibitor of NF-κB, IκBα, nuclear translocation of the NF-κB p65 subunit, and increased NF-κB transcriptional activity. Treatment of THP-1 macrophages with rHSP27 yielded increased expression of a variety of genes, including the pro-inflammatory factors, IL-1β, and TNF-α. However, rHSP27 also increased the expression of the anti-inflammatory factors IL-10 and GM-CSF both at the mRNA and protein levels. Our study suggests that in macrophages, activation of NF-κB signaling by rHSP27 is associated with upregulated expression and secretion of key pro- and anti-inflammatory cytokines. Moreover, we surmise that it is the balance in expression of these mediators and antagonists of inflammation, and hence atherogenesis, that yields a favorable net effect of HSP27 on the vessel wall.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

