Dynamics of target-mediated drug disposition: characteristic profiles and parameter identification
- PMID: 22851162
- PMCID: PMC3446204
- DOI: 10.1007/s10928-012-9260-6
Dynamics of target-mediated drug disposition: characteristic profiles and parameter identification
Abstract
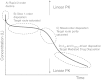
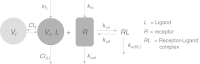
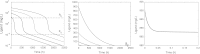
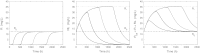
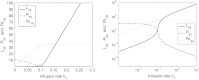
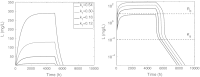
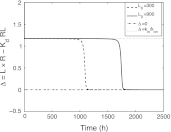
In this paper we present a mathematical analysis of the basic model for target mediated drug disposition (TMDD). Assuming high affinity of ligand to target, we give a qualitative characterisation of ligand versus time graphs for different dosing regimes and derive accurate analytic approximations of different phases in the temporal behaviour of the system. These approximations are used to estimate model parameters, give analytical approximations of such quantities as area under the ligand curve and clearance. We formulate conditions under which a suitably chosen Michaelis-Menten model provides a good approximation of the full TMDD-model over a specified time interval.
Figures










Similar articles
-
Dynamics of target-mediated drug disposition.Eur J Pharm Sci. 2009 Dec 8;38(5):445-64. doi: 10.1016/j.ejps.2009.09.007. Epub 2009 Sep 26. Eur J Pharm Sci. 2009. PMID: 19786099
-
Dose correction for the Michaelis-Menten approximation of the target-mediated drug disposition model.J Pharmacokinet Pharmacodyn. 2012 Apr;39(2):141-6. doi: 10.1007/s10928-011-9233-1. Epub 2012 Jan 4. J Pharmacokinet Pharmacodyn. 2012. PMID: 22215144 Free PMC article.
-
Numerical validation and properties of a rapid binding approximation of a target-mediated drug disposition pharmacokinetic model.J Pharmacokinet Pharmacodyn. 2009 Jun;36(3):199-219. doi: 10.1007/s10928-009-9118-8. Epub 2009 May 12. J Pharmacokinet Pharmacodyn. 2009. PMID: 19434483
-
Target-mediated drug disposition model: approximations, identifiability of model parameters and applications to the population pharmacokinetic-pharmacodynamic modeling of biologics.Expert Opin Drug Metab Toxicol. 2009 Jul;5(7):803-12. doi: 10.1517/17425250902992901. Expert Opin Drug Metab Toxicol. 2009. PMID: 19505189 Review.
-
Small-Molecule Compounds Exhibiting Target-Mediated Drug Disposition (TMDD): A Minireview.J Clin Pharmacol. 2017 Feb;57(2):137-150. doi: 10.1002/jcph.804. Epub 2016 Sep 6. J Clin Pharmacol. 2017. PMID: 27489162 Review.
Cited by
-
Target-mediated drug disposition with drug-drug interaction, Part I: single drug case in alternative formulations.J Pharmacokinet Pharmacodyn. 2017 Feb;44(1):17-26. doi: 10.1007/s10928-016-9501-1. Epub 2017 Jan 10. J Pharmacokinet Pharmacodyn. 2017. PMID: 28074395 Free PMC article.
-
Therapeutic drug monitoring of immune checkpoint inhibitors: based on their pharmacokinetic properties and biomarkers.Cancer Chemother Pharmacol. 2023 Sep;92(3):165-179. doi: 10.1007/s00280-023-04541-8. Epub 2023 Jul 6. Cancer Chemother Pharmacol. 2023. PMID: 37410155 Review.
-
Impact of mathematical pharmacology on practice and theory: four case studies.J Pharmacokinet Pharmacodyn. 2018 Feb;45(1):3-21. doi: 10.1007/s10928-017-9539-8. Epub 2017 Sep 7. J Pharmacokinet Pharmacodyn. 2018. PMID: 28884259 Free PMC article.
-
Pharmacokinetic and pharmacodynamic considerations for the next generation protein therapeutics.J Pharmacokinet Pharmacodyn. 2015 Oct;42(5):553-71. doi: 10.1007/s10928-015-9447-8. Epub 2015 Sep 15. J Pharmacokinet Pharmacodyn. 2015. PMID: 26373957 Free PMC article. Review.
-
A Tutorial on Target-Mediated Drug Disposition (TMDD) Models.CPT Pharmacometrics Syst Pharmacol. 2015 Jun;4(6):324-37. doi: 10.1002/psp4.41. Epub 2015 Jun 15. CPT Pharmacometrics Syst Pharmacol. 2015. PMID: 26225261 Free PMC article.
References
-
- Michaelis L, Menten ML. Die Kinetik der Invertinwirkung. Biochem Z. 1913;49:333–369.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

