Glycosphingolipids are essential for intestinal endocytic function
- PMID: 22851168
- PMCID: PMC3463339
- DOI: 10.1074/jbc.M112.371005
Glycosphingolipids are essential for intestinal endocytic function
Abstract
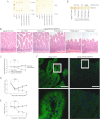
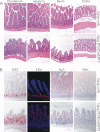
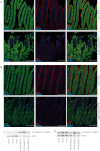
Glycosphingolipids (GSLs) constitute major components of enterocytes and were hypothesized to be potentially important for intestinal epithelial polarization. The enzyme UDP-glucose ceramide glucosyltransferase (Ugcg) catalyzes the initial step of GSL biosynthesis. Newborn and adult mice with enterocyte-specific genetic deletion of the gene Ugcg were generated. In newborn mutants lacking GSLs at day P0, intestinal epithelia were indistinguishable from those in control littermates displaying an intact polarization with regular brush border. However, those mice were not consistently able to absorb nutritional lipids from milk. Between postnatal days 5 and 7, severe defects in intestinal epithelial differentiation occurred accompanied by impaired intestinal uptake of nutrients. Villi of mutant mice became stunted, and enterocytes lacked brush border. The defects observed in mutant mice caused diarrhea, malabsorption, and early death. In this study, we show that GSLs are essential for enterocyte resorptive function but are primarily not for polarization; GSLs are required for intracellular vesicular transport in resorption-active intestine.
Figures










Similar articles
-
Cell-specific in vivo functions of glycosphingolipids: lessons from genetic deletions of enzymes involved in glycosphingolipid synthesis.Prog Lipid Res. 2013 Apr;52(2):231-48. doi: 10.1016/j.plipres.2013.02.001. Epub 2013 Mar 5. Prog Lipid Res. 2013. PMID: 23473748 Review.
-
Hepatic glycosphingolipid deficiency and liver function in mice.Hepatology. 2010 May;51(5):1799-809. doi: 10.1002/hep.23545. Hepatology. 2010. PMID: 20432257
-
Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth.Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12459-64. doi: 10.1073/pnas.0500893102. Epub 2005 Aug 18. Proc Natl Acad Sci U S A. 2005. PMID: 16109770 Free PMC article.
-
[Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth].Verh Dtsch Ges Pathol. 2006;90:193-202. Verh Dtsch Ges Pathol. 2006. PMID: 17867597 German.
-
Glucosylceramide synthase and glycosphingolipid synthesis.Trends Cell Biol. 1998 May;8(5):198-202. doi: 10.1016/s0962-8924(98)01249-5. Trends Cell Biol. 1998. PMID: 9695839 Review.
Cited by
-
Neuronal Ganglioside and Glycosphingolipid (GSL) Metabolism and Disease : Cascades of Secondary Metabolic Errors Can Generate Complex Pathologies (in LSDs).Adv Neurobiol. 2023;29:333-390. doi: 10.1007/978-3-031-12390-0_12. Adv Neurobiol. 2023. PMID: 36255681
-
1-O-acylceramides are natural components of human and mouse epidermis.J Lipid Res. 2013 Dec;54(12):3312-21. doi: 10.1194/jlr.M040097. Epub 2013 Sep 27. J Lipid Res. 2013. PMID: 24078707 Free PMC article.
-
Glucosylceramide Synthase Inhibition in Combination with Aripiprazole Sensitizes Hepatocellular Cancer Cells to Sorafenib and Doxorubicin.Int J Mol Sci. 2024 Dec 31;26(1):304. doi: 10.3390/ijms26010304. Int J Mol Sci. 2024. PMID: 39796160 Free PMC article.
-
Intestinal apical polarity mediates regulation of TORC1 by glucosylceramide in C. elegans.Genes Dev. 2015 Jun 15;29(12):1218-23. doi: 10.1101/gad.263483.115. Genes Dev. 2015. PMID: 26109047 Free PMC article.
-
Simplifying complexity: genetically resculpting glycosphingolipid synthesis pathways in mice to reveal function.Glycoconj J. 2014 Dec;31(9):613-22. doi: 10.1007/s10719-014-9563-5. Epub 2014 Oct 29. Glycoconj J. 2014. PMID: 25351657 Free PMC article. Review.
References
-
- Christiansen K., Carlsen J. (1981) Microvillus membrane vesicles from pig small intestine. Purity and lipid composition. Biochim. Biophys. Acta 647, 188–195 - PubMed
-
- Lingwood D., Simons K. (2010) Lipid rafts as a membrane-organizing principle. Science 327, 46–50 - PubMed
-
- Tamboli I. Y., Prager K., Barth E., Heneka M., Sandhoff K., Walter J. (2005) Inhibition of glycosphingolipid biosynthesis reduces secretion of the β-amyloid precursor protein and amyloid β-peptide. J. Biol. Chem. 280, 28110–28117 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

