PylSn and the homologous N-terminal domain of pyrrolysyl-tRNA synthetase bind the tRNA that is essential for the genetic encoding of pyrrolysine
- PMID: 22851181
- PMCID: PMC3463324
- DOI: 10.1074/jbc.M112.396754
PylSn and the homologous N-terminal domain of pyrrolysyl-tRNA synthetase bind the tRNA that is essential for the genetic encoding of pyrrolysine
Abstract
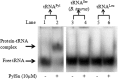
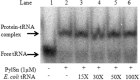
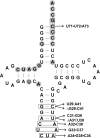
Pyrrolysine is represented by an amber codon in genes encoding proteins such as the methylamine methyltransferases present in some Archaea and Bacteria. Pyrrolysyl-tRNA synthetase (PylRS) attaches pyrrolysine to the amber-suppressing tRNA(Pyl). Archaeal PylRS, encoded by pylS, has a catalytic C-terminal domain but an N-terminal region of unknown function and structure. In Bacteria, homologs of the N- and C-terminal regions of archaeal PylRS are respectively encoded by pylSn and pylSc. We show here that wild type PylS from Methanosarcina barkeri and PylSn from Desulfitobacterium hafniense bind tRNA(Pyl) in EMSA with apparent K(d) values of 0.12 and 0.13 μM, respectively. Truncation of the N-terminal region of PylS eliminated detectable tRNA(Pyl) binding as measured by EMSA, but not catalytic activity. A chimeric protein with PylSn fused to the N terminus of truncated PylS regained EMSA-detectable tRNA(Pyl) binding. PylSn did not bind other D. hafniense tRNAs, nor did the competition by the Escherichia coli tRNA pool interfere with tRNA(Pyl) binding. Further indicating the specificity of PylSn interaction with tRNA(Pyl), substitutions of conserved residues in tRNA(Pyl) in the variable loop, D stem, and T stem and loop had significant impact in binding, whereas those having base changes in the acceptor stem or anticodon stem and loop still retained the ability to complex with PylSn. PylSn and the N terminus of PylS comprise the protein superfamily TIGR03129. The members of this family are not similar to any known RNA-binding protein, but our results suggest their common function involves specific binding of tRNA(Pyl).
Figures





References
-
- Atkins J. F., Gesteland R. (2002) The 22nd amino acid. Science 296, 1409–1410 - PubMed
-
- Krzycki J. A. (2004) Function of genetically encoded pyrrolysine in corrinoid-dependent methylamine methyltransferases. Curr. Opin. Chem. Biol. 8, 484–491 - PubMed
-
- Hao B., Gong W., Ferguson T. K., James C. M., Krzycki J. A., Chan M. K. (2002) A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science 296, 1462–1466 - PubMed
-
- Soares J. A., Zhang L., Pitsch R. L., Kleinholz N. M., Jones R. B., Wolff J. J., Amster J., Green-Church K. B., Krzycki J. A. (2005) The residue mass of l-pyrrolysine in three distinct methylamine methyltransferases. J. Biol. Chem. 280, 36962–36969 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

