Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration
- PMID: 22851518
- PMCID: PMC3448815
- DOI: 10.1002/stem.1186
Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration
Abstract
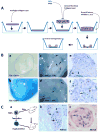
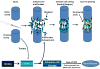
Peripheral nerves have the potential to regenerate axons and reinnervate end organs. Chronic denervation and disturbed nerve regeneration are thought to contribute to peripheral neuropathy, pain, and pruritus in the skin. The capacity of denervated distal nerves to support axonal regeneration requires proliferation by Schwann cells, which guide regenerating axons to their denervated targets. However, adult peripheral nerve Schwann cells do not retain a growth-permissive phenotype, as is required to produce new glia. Therefore, it is believed that following injury, mature Schwann cells dedifferentiate to a progenitor/stem cell phenotype to promote axonal regrowth. In this study, we show that skin-derived precursors (SKPs), a recently identified neural crest-related stem cell population in the dermis of skin, are an alternative source of progenitors for cutaneous nerve regeneration. Using in vivo and in vitro three-dimensional cutaneous nerve regeneration models, we show that the SKPs are neurotropic toward injured nerves and that they have a full capacity to differentiate into Schwann cells and promote axon regeneration. The identification of SKPs as a physiologic source of progenitors for cutaneous nerve regeneration in the skin, where SKPs physiologically reside, has important implications for understanding early cellular events in peripheral nerve regeneration. It also provides fertile ground for the elucidation of intrinsic and extrinsic factors within the nerve microenvironment that likely play essential roles in cutaneous nerve homeostasis.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
The authors have no potential conflicts of interest.
Figures




Similar articles
-
Practical considerations concerning the use of stem cells for peripheral nerve repair.Neurosurg Focus. 2009 Feb;26(2):E2. doi: 10.3171/FOC.2009.26.2.E2. Neurosurg Focus. 2009. PMID: 19435443 Review.
-
Use of stem cells to augment nerve injury repair.Neurosurgery. 2009 Oct;65(4 Suppl):A80-6. doi: 10.1227/01.NEU.0000335651.93926.2F. Neurosurgery. 2009. PMID: 19927083 Review.
-
Transforming growth factor-beta and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo.Glia. 2002 Mar 1;37(3):206-18. doi: 10.1002/glia.10022. Glia. 2002. PMID: 11857679
-
Adult skin-derived precursor Schwann cells exhibit superior myelination and regeneration supportive properties compared to chronically denervated nerve-derived Schwann cells.Exp Neurol. 2016 Apr;278:127-42. doi: 10.1016/j.expneurol.2016.02.006. Epub 2016 Feb 6. Exp Neurol. 2016. PMID: 26854934
-
Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation.Exp Neurol. 2010 May;223(1):221-8. doi: 10.1016/j.expneurol.2009.05.025. Epub 2009 May 27. Exp Neurol. 2010. PMID: 19477174
Cited by
-
UVRAG Promotes Tumor Progression through Regulating SP1 in Colorectal Cancer.Cancers (Basel). 2023 Apr 27;15(9):2502. doi: 10.3390/cancers15092502. Cancers (Basel). 2023. PMID: 37173968 Free PMC article.
-
The regulation of the homeostasis and regeneration of peripheral nerve is distinct from the CNS and independent of a stem cell population.Development. 2018 Dec 14;145(24):dev170316. doi: 10.1242/dev.170316. Development. 2018. PMID: 30413560 Free PMC article.
-
Spatiotemporal Loss of NF1 in Schwann Cell Lineage Leads to Different Types of Cutaneous Neurofibroma Susceptible to Modification by the Hippo Pathway.Cancer Discov. 2019 Jan;9(1):114-129. doi: 10.1158/2159-8290.CD-18-0151. Epub 2018 Oct 22. Cancer Discov. 2019. PMID: 30348677 Free PMC article.
-
Cellular Heterogeneity Facilitates the Functional Differences Between Hair Follicle Dermal Sheath Cells and Dermal Papilla Cells: A New Classification System for Mesenchymal Cells within the Hair Follicle Niche.Stem Cell Rev Rep. 2022 Aug;18(6):2016-2027. doi: 10.1007/s12015-022-10411-2. Epub 2022 Jul 18. Stem Cell Rev Rep. 2022. PMID: 35849252 Review.
-
The Effect of Liraglutide on Axon Regeneration and Functional Recovery after Peripheral Nerve Lesion.Curr Issues Mol Biol. 2024 Jan 2;46(1):327-339. doi: 10.3390/cimb46010021. Curr Issues Mol Biol. 2024. PMID: 38248323 Free PMC article.
References
-
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. - PubMed
-
- Zochodne DW. Neurobiology of Peripheral Nerve Regeneration. New York: Cambridge University Press; 2008.
-
- Rosemberg S, Marie SK, Kliemann S. Congenital insensitivity to pain with anhidrosis (hereditary sensory and autonomic neuropathy type IV) Pediatr Neurol. 1994;11:50–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

