Tracking chromatid segregation to identify human cardiac stem cells that regenerate extensively the infarcted myocardium
- PMID: 22851539
- PMCID: PMC3482833
- DOI: 10.1161/CIRCRESAHA.112.273649
Tracking chromatid segregation to identify human cardiac stem cells that regenerate extensively the infarcted myocardium
Erratum in
-
Correction.Circ Res. 2015 Dec 4;117(12):e131. doi: 10.1161/RES.0000000000000086. Circ Res. 2015. PMID: 26635385 No abstract available.
Retraction in
-
Retraction of: Tracking Chromatid Segregation to Identify Human Cardiac Stem Cells That Regenerate Extensively the Infarcted Myocardium.Circ Res. 2019 Feb 15;124(4):e29. doi: 10.1161/RES.0000000000000253. Circ Res. 2019. PMID: 30582468 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.Circ Res. 2019 Jan 18;124(2):e4-e5. doi: 10.1161/RES.0000000000000241. Circ Res. 2019. PMID: 30582460 No abstract available.
-
Expression of Concern.Circulation. 2019 Jan 15;139(3):e5-e6. doi: 10.1161/CIR.0000000000000639. Circulation. 2019. PMID: 30615475 No abstract available.
Abstract
Rationale: According to the immortal DNA strand hypothesis, dividing stem cells selectively segregate chromosomes carrying the old template DNA, opposing accumulation of mutations resulting from nonrepaired replication errors and attenuating telomere shortening.
Objective: Based on the premise of the immortal DNA strand hypothesis, we propose that stem cells retaining the old DNA would represent the most powerful cells for myocardial regeneration.
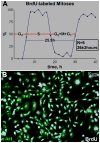
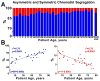
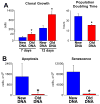
Methods and results: Division of human cardiac stem cells (hCSCs) by nonrandom and random segregation of chromatids was documented by clonal assay of bromodeoxyuridine-tagged hCSCs. Additionally, their growth properties were determined by a series of in vitro and in vivo studies. We report that a small class of hCSCs retain during replication the mother DNA and generate 2 daughter cells, which carry the old and new DNA, respectively. hCSCs with immortal DNA form a pool of nonsenescent cells with longer telomeres and higher proliferative capacity. The self-renewal and long-term repopulating ability of these cells was shown in serial-transplantation assays in the infarcted heart; these cells created a chimeric organ, composed of spared rat and regenerated human cardiomyocytes and coronary vessels, leading to a remarkable restoration of cardiac structure and function. The documentation that hCSCs divide by asymmetrical and symmetrical chromatid segregation supports the view that the human heart is a self-renewing organ regulated by a compartment of resident hCSCs.
Conclusions: The impressive recovery in ventricular hemodynamics and anatomy mediated by clonal hCSCs carrying the "mother" DNA underscores the clinical relevance of this stem cell class for the management of heart failure in humans.
Figures















Comment in
-
Biased DNA segregation and cardiac stem cell therapies.Circ Res. 2012 Sep 14;111(7):827-30. doi: 10.1161/CIRCRESAHA.112.277764. Circ Res. 2012. PMID: 22982871 No abstract available.
Similar articles
-
Biased DNA segregation and cardiac stem cell therapies.Circ Res. 2012 Sep 14;111(7):827-30. doi: 10.1161/CIRCRESAHA.112.277764. Circ Res. 2012. PMID: 22982871 No abstract available.
-
A Novel Class of Human Cardiac Stem Cells.Cardiol Rev. 2015 Jul-Aug;23(4):189-200. doi: 10.1097/CRD.0000000000000064. Cardiol Rev. 2015. PMID: 25807105 Free PMC article.
-
Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration.Circ Res. 2011 Jun 10;108(12):1467-81. doi: 10.1161/CIRCRESAHA.111.240648. Epub 2011 May 5. Circ Res. 2011. Retraction in: Circ Res. 2019 Feb 15;124(4):e26. doi: 10.1161/RES.0000000000000250. PMID: 21546606 Free PMC article. Retracted.
-
Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration.Nat Clin Pract Cardiovasc Med. 2006 Mar;3 Suppl 1:S8-13. doi: 10.1038/ncpcardio0409. Nat Clin Pract Cardiovasc Med. 2006. PMID: 16501638 Review.
-
Stem cell identity and template DNA strand segregation.Curr Opin Cell Biol. 2008 Dec;20(6):716-22. doi: 10.1016/j.ceb.2008.10.004. Epub 2008 Nov 25. Curr Opin Cell Biol. 2008. PMID: 18996191 Review.
Cited by
-
Cardiac stem cells: biology and clinical applications.Antioxid Redox Signal. 2014 Nov 10;21(14):2002-17. doi: 10.1089/ars.2014.5875. Epub 2014 Apr 10. Antioxid Redox Signal. 2014. PMID: 24597850 Free PMC article. Review.
-
Stem cells and myocardial regeneration: cooperation wins over competition.Circulation. 2013 Jan 15;127(2):165-8. doi: 10.1161/CIRCULATIONAHA.112.153973. Epub 2012 Dec 5. Circulation. 2013. PMID: 23224060 Free PMC article. No abstract available.
-
Endogenous cardiac stem cells for the treatment of heart failure.Stem Cells Cloning. 2013 Mar 25;6:1-12. doi: 10.2147/SCCAA.S29221. Stem Cells Cloning. 2013. PMID: 24426784 Free PMC article. Review.
-
Symmetry from Asymmetry or Asymmetry from Symmetry?Cold Spring Harb Symp Quant Biol. 2017;82:305-318. doi: 10.1101/sqb.2017.82.034272. Epub 2018 Jan 18. Cold Spring Harb Symp Quant Biol. 2017. PMID: 29348326 Free PMC article.
-
Origin of cardiomyocytes in the adult heart.Circ Res. 2015 Jan 2;116(1):150-66. doi: 10.1161/CIRCRESAHA.116.303595. Circ Res. 2015. PMID: 25552694 Free PMC article. Review.
References
-
- Murry CE, Lee RT. Development biology. Turnover after the fallout. Science. 2009;324:47–48. - PubMed
-
- Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. - PubMed
-
- Bayes-Genis A, Roura S, Prat-Vidal C, Farré J, Soler-Botija C, de Luna AB, Cinca J. Chimerism and microchimerism of the human heart: evidence for cardiac regeneration. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S40–S45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL111183/HL/NHLBI NIH HHS/United States
- R01 HL075480/HL/NHLBI NIH HHS/United States
- R01 HL114346/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 HL105532/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- P01 HL078825/HL/NHLBI NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

