Phase II study of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone immunochemotherapy followed by yttrium-90-ibritumomab tiuxetan in untreated mantle-cell lymphoma: Eastern Cooperative Oncology Group Study E1499
- PMID: 22851557
- PMCID: PMC3732008
- DOI: 10.1200/JCO.2012.42.2444
Phase II study of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone immunochemotherapy followed by yttrium-90-ibritumomab tiuxetan in untreated mantle-cell lymphoma: Eastern Cooperative Oncology Group Study E1499
Abstract
Purpose: To test the hypothesis that consolidation therapy with yttrium-90 ((90)Y) -ibritumomab tiuxetan after brief initial therapy with four cycles of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with previously untreated mantle-cell lymphoma would be a well-tolerated regimen that would improve outcomes compared with historical R-CHOP data.
Patients and methods: Patients ≥ 18 years old with histologically confirmed mantle-cell lymphoma expressing CD20 and cyclin D1 who had not received any previous therapy and had an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate organ function were eligible. The study enrolled and treated 57 patients, of whom 56 patients were eligible. Fifty-two patients (50 eligible patients) received (90)Y-ibritumomab tiuxetan. The study design required 52 eligible patients to detect a 50% improvement in the median time to treatment failure (TTF) compared with that reported for six cycles of R-CHOP.
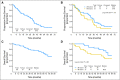
Results: With 56 analyzed patients (median age, 60 years; men, 73%), the overall response rate was 82% (55% complete response/complete response-unconfirmed). With a median follow-up of 72 months, the median TTF was 34.2 months. The median overall survival (OS) has not been reached, with an estimated 5-year OS of 73% (79% for patients ≤ age 65 years v 62% for patients > age 65 years; P = .08 [log-rank test]). There were no unexpected toxicities.
Conclusion: R-CHOP given for four cycles followed by (90)Y-ibritumomab tiuxetan compared favorably with historical results with six cycles of R-CHOP in patients with previously untreated mantle-cell lymphoma. This regimen was well tolerated and should be applicable to most patients with this disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study.Lancet Oncol. 2018 Nov;19(11):1449-1458. doi: 10.1016/S1470-2045(18)30685-5. Epub 2018 Oct 19. Lancet Oncol. 2018. PMID: 30348538 Clinical Trial.
-
Short duration immunochemotherapy followed by radioimmunotherapy consolidation is effective and well tolerated in relapsed follicular lymphoma: 5-year results from a UK National Cancer Research Institute Lymphoma Group study.Br J Haematol. 2016 Apr;173(2):274-82. doi: 10.1111/bjh.13954. Epub 2016 Feb 5. Br J Haematol. 2016. PMID: 26849853 Clinical Trial.
-
Phase II trial of short-course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients.Clin Cancer Res. 2010 Aug 1;16(15):3998-4004. doi: 10.1158/1078-0432.CCR-10-0162. Epub 2010 Jun 11. Clin Cancer Res. 2010. PMID: 20542986 Clinical Trial.
-
Immunotherapy with rituximab following high-dose therapy and autologous stem-cell transplantation for mantle cell lymphoma.Semin Oncol. 2002 Feb;29(1 Suppl 2):56-69. Semin Oncol. 2002. PMID: 11842390 Review.
-
Cardiovascular adverse events in patients with non-Hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (R-CHOP): a systematic review and meta-analysis.Lancet Haematol. 2020 Apr;7(4):e295-e308. doi: 10.1016/S2352-3026(20)30031-4. Epub 2020 Mar 2. Lancet Haematol. 2020. PMID: 32135128
Cited by
-
Radioimmunotherapy for mantle cell lymphoma: 5-year follow-up of 90 patients from the international RIT registry.Ann Hematol. 2020 May;99(5):1073-1079. doi: 10.1007/s00277-020-03956-0. Epub 2020 Mar 3. Ann Hematol. 2020. PMID: 32125469 Free PMC article. Clinical Trial.
-
Racial, ethnic, sex, and age representation in clinical trials of radiopharmaceutical tumor therapy: a systematic review and meta-analysis.BMC Cancer. 2025 Jul 1;25(1):1092. doi: 10.1186/s12885-025-14527-9. BMC Cancer. 2025. PMID: 40597097 Free PMC article.
-
Frontline Treatment for Older Patients with Mantle Cell Lymphoma.Oncologist. 2018 Nov;23(11):1337-1348. doi: 10.1634/theoncologist.2017-0470. Epub 2018 Jun 12. Oncologist. 2018. PMID: 29895632 Free PMC article. Review.
-
Radioimmunotherapy of B-Cell Non-Hodgkin's Lymphoma.Front Oncol. 2013 Jul 11;3:177. doi: 10.3389/fonc.2013.00177. eCollection 2013. Front Oncol. 2013. PMID: 23875170 Free PMC article.
-
Mantle Cell Lymphoma: First-line Therapy in Patients Not Eligible for Stem Cell Transplantation.Curr Treat Options Oncol. 2015 Jun;16(6):29. doi: 10.1007/s11864-015-0343-7. Curr Treat Options Oncol. 2015. PMID: 25975443 Review.
References
-
- Harris NL, Jaffé ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: A proposal from the international lymphoma study group. Blood. 1994;84:1361–1392. - PubMed
-
- Williams ME, Swerdlow SH. Cyclin D1 overexpression in non-Hodgkin's lymphoma with chromosome 11 bcl-1 rearrangement. Ann Oncol. 1994;5:71–73. - PubMed
-
- Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: Molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288–1294. - PubMed
-
- Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. - PubMed
-
- Romaguera JE, Fayad LE, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA21076/CA/NCI NIH HHS/United States
- U10 CA027525/CA/NCI NIH HHS/United States
- CA14958/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA014958/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- CA27525/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

