Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial
- PMID: 22851600
- PMCID: PMC3447855
- DOI: 10.2337/dc11-2113
Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial
Abstract
Objective: To assess the efficacy and safety of switching from sitagliptin to liraglutide in metformin-treated adults with type 2 diabetes.
Research design and methods: In an open-label trial, participants randomized to receive either liraglutide (1.2 or 1.8 mg/day) or sitagliptin (100 mg/day), each added to metformin, continued treatment for 52 weeks. In a 26-week extension, sitagliptin-treated participants were randomly allocated to receive instead liraglutide at either 1.2 or 1.8 mg/day, while participants originally randomized to receive liraglutide continued unchanged.
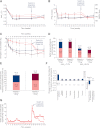
Results: Although 52 weeks of sitagliptin changed glycosylated hemoglobin (HbA(1c)) by -0.9% from baseline, additional decreases occurred after switching to liraglutide (1.2 mg/day, -0.2%, P = 0.006; 1.8 mg/day, -0.5%, P = 0.0001). Conversion to liraglutide was associated with reductions in fasting plasma glucose (FPG) (1.2 mg/day, -0.8 mmol/L, P = 0.0004; 1.8 mg/day, -1.4 mmol/L, P < 0.0001) and body weight (1.2 mg/day, -1.6 kg; 1.8 mg/day, -2.5 kg; both P < 0.0001) and with an increased proportion of patients reaching HbA(1c) <7% (from ∼30% to ∼50%). Overall treatment satisfaction, assessed by the Diabetes Treatment Satisfaction Questionnaire, improved after switching to liraglutide (pooled 1.2 and 1.8 mg/day, 1.3; P = 0.0189). After switching, mostly transient nausea occurred in 21% of participants, and minor hypoglycemia remained low (3-4% of participants). Continuing liraglutide treatment at 1.2 mg/day and 1.8 mg/day for 78 weeks reduced HbA(1c) (baseline 8.3 and 8.4%, respectively) by -0.9 and -1.3%, respectively; FPG by -1.3 and -1.7 mmol/L, respectively; and weight by -2.6 and -3.1 kg, respectively, with 9-10% of participants reporting minor hypoglycemia.
Conclusions: Glycemic control, weight, and treatment satisfaction improved after switching from sitagliptin to liraglutide, albeit with a transient increase in gastrointestinal reactions.
Figures

References
-
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab 2009;11(Suppl. 3):26–34 - PubMed
-
- Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 - PubMed
-
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 - PubMed
-
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 - PubMed
-
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

