Clonal interference in the evolution of influenza
- PMID: 22851649
- PMCID: PMC3454888
- DOI: 10.1534/genetics.112.143396
Clonal interference in the evolution of influenza
Abstract
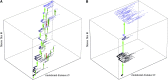
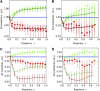
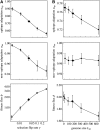
The seasonal influenza A virus undergoes rapid evolution to escape human immune response. Adaptive changes occur primarily in antigenic epitopes, the antibody-binding domains of the viral hemagglutinin. This process involves recurrent selective sweeps, in which clusters of simultaneous nucleotide fixations in the hemagglutinin coding sequence are observed about every 4 years. Here, we show that influenza A (H3N2) evolves by strong clonal interference. This mode of evolution is a red queen race between viral strains with different beneficial mutations. Clonal interference explains and quantifies the observed sweep pattern: we find an average of at least one strongly beneficial amino acid substitution per year, and a given selective sweep has three to four driving mutations on average. The inference of selection and clonal interference is based on frequency time series of single-nucleotide polymorphisms, which are obtained from a sample of influenza genome sequences over 39 years. Our results imply that mode and speed of influenza evolution are governed not only by positive selection within, but also by background selection outside antigenic epitopes: immune adaptation and conservation of other viral functions interfere with each other. Hence, adapting viral proteins are predicted to be particularly brittle. We conclude that a quantitative understanding of influenza's evolutionary and epidemiological dynamics must be based on all genomic domains and functions coupled by clonal interference.
Figures


References
-
- Bolthausen E., Sznitman A. S., 1998. On Ruelle’s probability cascades and an abstract cavity method. Commun. Math. Phys. 197: 247–276
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

