Emergence of fatal avian influenza in New England harbor seals
- PMID: 22851656
- PMCID: PMC3419516
- DOI: 10.1128/mBio.00166-12
Emergence of fatal avian influenza in New England harbor seals
Abstract
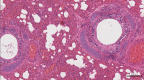
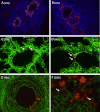
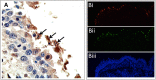
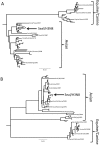
From September to December 2011, 162 New England harbor seals died in an outbreak of pneumonia. Sequence analysis of postmortem samples revealed the presence of an avian H3N8 influenza A virus, similar to a virus circulating in North American waterfowl since at least 2002 but with mutations that indicate recent adaption to mammalian hosts. These include a D701N mutation in the viral PB2 protein, previously reported in highly pathogenic H5N1 avian influenza viruses infecting people. Lectin staining and agglutination assays indicated the presence of the avian-preferred SAα-2,3 and mammalian SAα-2,6 receptors in seal respiratory tract, and the ability of the virus to agglutinate erythrocytes bearing either the SAα-2,3 or the SAα-2,6 receptor. The emergence of this A/harbor seal/Massachusetts/1/2011 virus may herald the appearance of an H3N8 influenza clade with potential for persistence and cross-species transmission.
Importance: The emergence of new strains of influenza virus is always of great public concern, especially when the infection of a new mammalian host has the potential to result in a widespread outbreak of disease. Here we report the emergence of an avian influenza virus (H3N8) in New England harbor seals which caused an outbreak of pneumonia and contributed to a U.S. federally recognized unusual mortality event (UME). This outbreak is particularly significant, not only because of the disease it caused in seals but also because the virus has naturally acquired mutations that are known to increase transmissibility and virulence in mammals. Monitoring the spillover and adaptation of avian viruses in mammalian species is critically important if we are to understand the factors that lead to both epizootic and zoonotic emergence.
Figures

References
-
- Geraci JR, et al. 1982. Mass mortality of harbor seals: pneumonia associated with influenza A virus. Science 215:1129–1131 - PubMed
-
- Webster RG, et al. 1981. Characterization of an influenza A virus from seals. Virology 113:712–724 - PubMed
-
- Callan RJ, Early G, Kida H, Hinshaw VS. 1995. The appearance of H3 influenza viruses in seals. J. Gen. Virol. 76(Part 1):199–203 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
