Sequential eukaryotic translation initiation factor 5 (eIF5) binding to the charged disordered segments of eIF4G and eIF2β stabilizes the 48S preinitiation complex and promotes its shift to the initiation mode
- PMID: 22851688
- PMCID: PMC3457530
- DOI: 10.1128/MCB.00376-12
Sequential eukaryotic translation initiation factor 5 (eIF5) binding to the charged disordered segments of eIF4G and eIF2β stabilizes the 48S preinitiation complex and promotes its shift to the initiation mode
Abstract
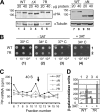
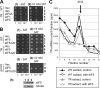
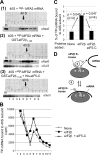
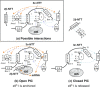
During translation initiation in Saccharomyces cerevisiae, an Arg- and Ser-rich segment (RS1 domain) of eukaryotic translation initiation factor 4G (eIF4G) and the Lys-rich segment (K-boxes) of eIF2β bind three common partners, eIF5, eIF1, and mRNA. Here, we report that both of these segments are involved in mRNA recruitment and AUG recognition by distinct mechanisms. First, the eIF4G-RS1 interaction with the eIF5 C-terminal domain (eIF5-CTD) directly links eIF4G to the preinitiation complex (PIC) and enhances mRNA binding. Second, eIF2β-K-boxes increase mRNA binding to the 40S subunit in vitro in a manner reversed by the eIF5-CTD. Third, mutations altering eIF4G-RS1, eIF2β-K-boxes, and eIF5-CTD restore the accuracy of start codon selection impaired by an eIF2β mutation in vivo, suggesting that the mutual interactions of the eIF segments within the PIC prime the ribosome for initiation in response to start codon selection. We propose that the rearrangement of interactions involving the eIF5-CTD promotes mRNA recruitment through mRNA binding by eIF4G and eIF2β and assists the start codon-induced release of eIF1, the major antagonist of establishing tRNA(i)(Met):mRNA binding to the P site.
Figures



References
-
- Acker MG, Kolitz SE, Mitchell SF, Nanda JS, Lorsch JR. 2007. Reconstitution of yeast translation initiation. Methods Enzymol. 430: 111– 145 - PubMed
-
- Algire MA, Maag D, Lorsch JR. 2005. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20: 251– 262 - PubMed
-
- Asano K, et al. 2002. Analysis and reconstitution of translation initiation in vitro. Methods Enzymol. 351: 221– 247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
