Expression profiling during mammary epithelial cell three-dimensional morphogenesis identifies PTPRO as a novel regulator of morphogenesis and ErbB2-mediated transformation
- PMID: 22851698
- PMCID: PMC3457532
- DOI: 10.1128/MCB.00068-12
Expression profiling during mammary epithelial cell three-dimensional morphogenesis identifies PTPRO as a novel regulator of morphogenesis and ErbB2-mediated transformation
Abstract
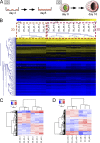
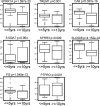
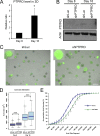
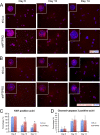
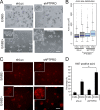
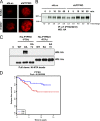
Identification of genes that are upregulated during mammary epithelial cell morphogenesis may reveal novel regulators of tumorigenesis. We have demonstrated that gene expression programs in mammary epithelial cells grown in monolayer cultures differ significantly from those in three-dimensional (3D) cultures. We identify a protein tyrosine phosphate, PTPRO, that was upregulated in mature MCF-10A mammary epithelial 3D structures but had low to undetectable levels in monolayer cultures. Downregulation of PTPRO by RNA interference inhibited proliferation arrest during morphogenesis. Low levels of PTPRO expression correlated with reduced survival for breast cancer patients, suggesting a tumor suppressor function. Furthermore, we showed that the receptor tyrosine kinase ErbB2/HER2 is a direct substrate of PTPRO and that loss of PTPRO increased ErbB2-induced cell proliferation and transformation, together with tyrosine phosphorylation of ErbB2. Moreover, in patients with ErbB2-positive breast tumors, low PTPRO expression correlated with poor clinical prognosis compared to ErbB2-positive patients with high levels of PTPRO. Thus, PTPRO is a novel regulator of ErbB2 signaling, a potential tumor suppressor, and a novel prognostic marker for patients with ErbB2-positive breast cancers. We have identified the protein tyrosine phosphatase PTPRO as a regulator of three-dimensional epithelial morphogenesis of mammary epithelial cells and as a regulator of ErbB2-mediated transformation. In addition, we demonstrated that ErbB2 is a direct substrate of PTPRO and that decreased expression of PTPRO predicts poor prognosis for ErbB2-positive breast cancer patients. Thus, our results identify PTPRO as a novel regulator of mammary epithelial transformation, a potential tumor suppressor, and a predictive biomarker for breast cancer.
Figures
References
-
- Bentires-Alj M, Neel BG. 2007. Protein-tyrosine phosphatase 1B (PTP1B) is required for HER2/Neu-induced breast cancer. Cancer Res. 67:2420–2424 - PubMed
-
- Bild AH, et al. 2006. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439:353–357 - PubMed
-
- Chen L, Juszczynski P, Takeyama K, Aguiar RC, Shipp MA. 2006. Protein tyrosine phosphatase receptor-type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell-receptor signaling, and cellular proliferation. Blood 108:3428–3433 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
