An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo
- PMID: 22851709
- PMCID: PMC3633481
- DOI: 10.4049/jimmunol.1103495
An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo
Abstract
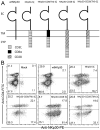
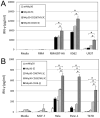
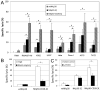
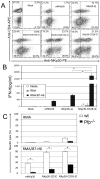
NKp30 is a natural cytotoxicity receptor that is expressed on NK cells and recognizes B7-H6, which is expressed on several types of tumors but few normal cells. To target effector T cells against B7-H6+ tumors, we developed several chimeric AgRs (CARs) based on NKp30, which contain the CD28- and/or CD3ζ-signaling domains with the transmembrane domains from CD3ζ, CD28, or CD8α. The data show that chimeric NKp30-expressing T cells responded to B7-H6+ tumor cells. The NKp30 CAR-expressing T cells produced IFN-γ and killed B7-H6 ligand-expressing tumor cells; this response was dependent upon ligand expression on target cells but not on MHC expression. PBMC-derived dendritic cells also express NKp30 ligands, including immature dendritic cells, and they can stimulate NKp30 CAR-bearing T cells to produce IFN-γ, but to a lesser extent. The addition of a CD28-signaling domain significantly enhanced the activity of the NKp30 CAR in a PI3K-dependent manner. Adoptive transfer of T cells expressing a chimeric NKp30 receptor containing a CD28-signaling domain inhibited the growth of a B7-H6-expressing murine lymphoma (RMA/B7-H6) in vivo. Moreover, mice that remained tumor-free were resistant to a subsequent challenge with the wild-type RMA tumor cells, suggesting the generation of immunity against other tumor Ags. Overall, this study demonstrates the specificity and therapeutic potential of adoptive immunotherapy with NKp30 CAR-expressing T cells against B7-H6+ tumor cells in vivo.
Figures



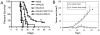
References
-
- Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10:5–18. - PubMed
-
- Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

