Structure-activity relationship of fenamates as Slo2.1 channel activators
- PMID: 22851714
- PMCID: PMC3477229
- DOI: 10.1124/mol.112.079194
Structure-activity relationship of fenamates as Slo2.1 channel activators
Abstract
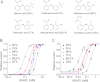
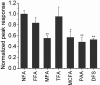
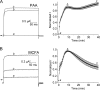
Niflumic acid, 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid (NFA), a nonsteroidal anti-inflammatory drug that blocks cyclooxygenase (COX), was shown previously to activate [Na(+)](i)-regulated Slo2.1 channels. In this study, we report that other fenamates, including flufenamic acid, mefenamic acid, tolfenamic acid, meclofenamic acid, and a phenyl acetic acid derivative, diclofenac, also are low-potency (EC(50) = 80 μM to 2.1 mM), partial agonists of human Slo2.1 channels heterologously expressed in Xenopus oocytes. Substituent analysis determined that N-phenylanthranilic acid was the minimal pharmacophore for fenamate activation of Slo2.1 channels. The effects of fenamates were biphasic, with an initial rapid activation phase followed by a slow phase of current inhibition. Ibuprofen, a structurally dissimilar COX inhibitor, did not activate Slo2.1. Preincubation of oocytes with ibuprofen did not significantly alter the effects of NFA, suggesting that neither channel activation nor inhibition is associated with COX activity. A point mutation (A278R) in the pore-lining S6 segment of Slo2.1 increased the sensitivity to activation and reduced the inhibition induced by NFA. Together, our results suggest that fenamates bind to two sites on Slo2.1 channels: an extracellular accessible site to activate and a cytoplasmic accessible site in the pore to inhibit currents.
Figures






Similar articles
-
Hydrophobic interactions between the S5 segment and the pore helix stabilizes the closed state of Slo2.1 potassium channels.Biochim Biophys Acta. 2016 Apr;1858(4):783-92. doi: 10.1016/j.bbamem.2015.12.024. Epub 2015 Dec 23. Biochim Biophys Acta. 2016. PMID: 26724206 Free PMC article.
-
Activation of Slo2.1 channels by niflumic acid.J Gen Physiol. 2010 Mar;135(3):275-95. doi: 10.1085/jgp.200910316. J Gen Physiol. 2010. PMID: 20176855 Free PMC article.
-
Identification of the Intracellular Na+ Sensor in Slo2.1 Potassium Channels.J Biol Chem. 2015 Jun 5;290(23):14528-35. doi: 10.1074/jbc.M115.653089. Epub 2015 Apr 22. J Biol Chem. 2015. PMID: 25903137 Free PMC article.
-
Degradation of fenamates.Profiles Drug Subst Excip Relat Methodol. 2025;50:229-275. doi: 10.1016/bs.podrm.2024.09.001. Epub 2024 Nov 2. Profiles Drug Subst Excip Relat Methodol. 2025. PMID: 39855777 Review.
-
Fenamates: Forgotten treasure for cancer treatment and prevention: Mechanisms of action, structural modification, and bright future.Med Res Rev. 2025 Jan;45(1):164-213. doi: 10.1002/med.22079. Epub 2024 Aug 22. Med Res Rev. 2025. PMID: 39171404 Review.
Cited by
-
Flufenamic acid as an ion channel modulator.Pharmacol Ther. 2013 May;138(2):272-84. doi: 10.1016/j.pharmthera.2013.01.012. Epub 2013 Jan 25. Pharmacol Ther. 2013. PMID: 23356979 Free PMC article. Review.
-
Molecular mechanisms of Slo2 K+ channel closure.J Physiol. 2017 Apr 1;595(7):2321-2336. doi: 10.1113/JP273225. Epub 2016 Dec 2. J Physiol. 2017. PMID: 27682982 Free PMC article.
-
Intracellular ATP does not inhibit Slo2.1 K+ channels.Physiol Rep. 2014 Sep 11;2(9):e12118. doi: 10.14814/phy2.12118. Print 2014 Sep 1. Physiol Rep. 2014. PMID: 25214519 Free PMC article.
-
The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection.Biochem J. 2017 Jun 9;474(12):2067-2094. doi: 10.1042/BCJ20160623. Biochem J. 2017. PMID: 28600454 Free PMC article. Review.
-
Hydrophobic interactions between the S5 segment and the pore helix stabilizes the closed state of Slo2.1 potassium channels.Biochim Biophys Acta. 2016 Apr;1858(4):783-92. doi: 10.1016/j.bbamem.2015.12.024. Epub 2015 Dec 23. Biochim Biophys Acta. 2016. PMID: 26724206 Free PMC article.
References
-
- Barnett J, Chow J, Ives D, Chiou M, Mackenzie R, Osen E, Nguyen B, Tsing S, Bach C, Freire J. (1994) Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta 1209:130–139 - PubMed
-
- Busch AE, Herzer T, Wagner CA, Schmidt F, Raber G, Waldegger S, Lang F. (1994) Positive regulation by chloride channel blockers of IsK channels expressed in Xenopus oocytes. Mol Pharmacol 46:750–753 - PubMed
-
- Dhanaraj V, Vijayan M. (1988) Structural studies of analgesics and their interactions. XII. Structure and interactions of anti-inflammatory fenamates. A concerted crystallographic and theoretical conformational study. Acta Crystallogr B 44:406–412 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

