Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset
- PMID: 22851756
- PMCID: PMC3457559
- DOI: 10.1128/IAI.00550-12
Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset
Abstract
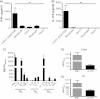
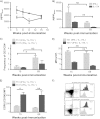
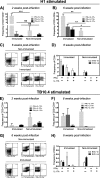
Th17 cells are increasingly being recognized as an important T helper subset for immune-mediated protection, especially against pathogens at mucosal ports of entry. In several cases, it would thus be highly relevant to induce Th17 memory by vaccination. Th17 cells are reported to exhibit high plasticity and may not stably maintain their differentiation program once induced, questioning the possibility of inducing durable Th17 memory. Accordingly, there is no consensus as to whether Th17 memory can be established unless influenced by continuous Th17 polarizing conditions. We have previously reported (T. Lindenstrøm, et al., J. Immunol. 182:8047-8055, 2009) that the cationic liposome adjuvant CAF01 can prime both Th1 and Th17 responses and promote robust, long-lived Th1 memory. Here, we demonstrate that subunit vaccination in mice with CAF01 leads to establishment of bona fide Th17 memory cells. Accordingly, Th17 memory cells exhibited lineage stability by retaining both phenotypic and functional properties for nearly 2 years. Antigen-specific, long-term Th17 memory cells were found to be mobilized from lung-draining lymph nodes to the lung following an aerosol challenge by Mycobacterium tuberculosis nearly 2 years after their induction and proliferated at levels comparable to those of Th1 memory cells. During the infection, the vaccine-induced Th17 memory cells expanded in the lungs and adapted Th1 characteristics, implying that they represent a metastable population which exhibits plasticity when exposed to prolonged Th1 polarizing, inflammatory conditions such as those found in the M. tuberculosis-infected lung. In the absence of overt inflammation, however, stable bona fide Th17 memory can indeed be induced by parenteral immunization.
Figures


References
-
- Aagaard C, et al. 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17:189–194 - PubMed
-
- Acosta-Rodriguez EV, et al. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646 - PubMed
-
- Agger EM, et al. 2008. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 3:e3116 doi:10.1371/journal.pone.0003116 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

