Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory
- PMID: 22851803
- PMCID: PMC3397788
- DOI: 10.5665/sleep.1992
Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory
Abstract
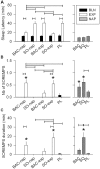
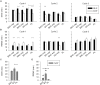
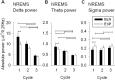
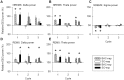
Study objectives: Sodium oxybate (SO) is a GABAβ agonist used to treat the sleep disorder narcolepsy. SO was shown to increase slow wave sleep (SWS) and EEG delta power (0.75-4.5 Hz), both indexes of NREM sleep (NREMS) intensity and depth, suggesting that SO enhances recuperative function of NREM. We investigated whether SO induces physiological deep sleep.
Design: SO was administered before an afternoon nap or before the subsequent experimental night in 13 healthy volunteers. The effects of SO were compared to baclofen (BAC), another GABAβ receptor agonist, to assess the role of GABAβ receptors in the SO response.
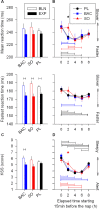
Measurements and results: As expected, a nap significantly decreased sleep need and intensity the subsequent night. Both drugs reversed this nap effect on the subsequent night by decreasing sleep latency and increasing total sleep time, SWS during the first NREMS episode, and EEG delta and theta (0.75-7.25 Hz) power during NREMS. The SO-induced increase in EEG delta and theta power was, however, not specific to NREMS and was also observed during REM sleep (REMS) and wakefulness. Moreover, the high levels of delta power during a nap following SO administration did not affect delta power the following night. SO and BAC taken before the nap did not improve subsequent psychomotor performance and subjective alertness, or memory consolidation. Finally, SO and BAC strongly promoted the appearance of sleep onset REM periods.
Conclusions: The SO-induced EEG slow waves seem not to be functionally similar to physiological slow waves. Our findings also suggest a role for GABAβ receptors in REMS generation.
Keywords: EEG delta activity; Narcolepsy; hypnotic; memory; sleep homeostasis.
Figures


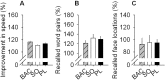
Similar articles
-
Baclofen and gamma-hydroxybutyrate differentially altered behavior, EEG activity and sleep in rats.Neuroscience. 2015 Jan 22;284:18-28. doi: 10.1016/j.neuroscience.2014.08.061. Epub 2014 Oct 6. Neuroscience. 2015. PMID: 25301745
-
Differential effects of GABAB receptor subtypes, {gamma}-hydroxybutyric Acid, and Baclofen on EEG activity and sleep regulation.J Neurosci. 2010 Oct 20;30(42):14194-204. doi: 10.1523/JNEUROSCI.3145-10.2010. J Neurosci. 2010. PMID: 20962240 Free PMC article.
-
A review of sodium oxybate and baclofen in the treatment of sleep disorders.Curr Pharm Des. 2011;17(15):1430-5. doi: 10.2174/138161211796197098. Curr Pharm Des. 2011. PMID: 21476957 Review.
-
The GABA(A) agonist gaboxadol improves the quality of post-nap sleep.Psychopharmacology (Berl). 2001 Sep;157(3):299-304. doi: 10.1007/s002130100819. Psychopharmacology (Berl). 2001. PMID: 11605086 Clinical Trial.
-
Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system.J Sleep Res. 2004 Sep;13(3):179-208. doi: 10.1111/j.1365-2869.2004.00412.x. J Sleep Res. 2004. PMID: 15339255 Review.
Cited by
-
Sleep restores behavioral plasticity to Drosophila mutants.Curr Biol. 2015 May 18;25(10):1270-81. doi: 10.1016/j.cub.2015.03.027. Epub 2015 Apr 23. Curr Biol. 2015. PMID: 25913403 Free PMC article.
-
Sleep disorders cause Parkinson's disease or the reverse is true: Good GABA good night.CNS Neurosci Ther. 2024 Mar;30(3):e14521. doi: 10.1111/cns.14521. CNS Neurosci Ther. 2024. PMID: 38491789 Free PMC article. Review.
-
The Use of Baclofen as a Treatment for Alcohol Use Disorder: A Clinical Practice Perspective.Front Psychiatry. 2019 Jan 4;9:708. doi: 10.3389/fpsyt.2018.00708. eCollection 2018. Front Psychiatry. 2019. PMID: 30662411 Free PMC article. Review.
-
Baclofen-associated neurophysiologic target engagement across species in fragile X syndrome.J Neurodev Disord. 2022 Sep 27;14(1):52. doi: 10.1186/s11689-022-09455-9. J Neurodev Disord. 2022. PMID: 36167501 Free PMC article. Clinical Trial.
-
Baclofen for narcolepsy with cataplexy: two cases.Nat Sci Sleep. 2015 Jul 29;7:81-3. doi: 10.2147/NSS.S86649. eCollection 2015. Nat Sci Sleep. 2015. PMID: 26251634 Free PMC article.
References
-
- U.S. Xyrem Multicenter Study Group. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–5. - PubMed
-
- Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–34. - PubMed
-
- Lapierre O, Montplaisir J, Lamarre M, Bedard MA. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: further considerations on REM sleep-triggering mechanisms. Sleep. 1990;13:24–30. - PubMed
-
- Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatol. 2003;30:1070–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

