Cellular and molecular mechanisms of intestinal fibrosis
- PMID: 22851857
- PMCID: PMC3406417
- DOI: 10.3748/wjg.v18.i28.3635
Cellular and molecular mechanisms of intestinal fibrosis
Abstract
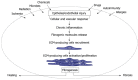
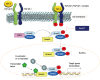
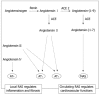
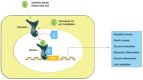
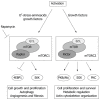
Fibrosis is a chronic and progressive process characterized by an excessive accumulation of extracellular matrix (ECM) leading to stiffening and/or scarring of the involved tissue. Intestinal fibrosis may develop in several different enteropathies, including inflammatory bowel disease. It develops through complex cell, extracellular matrix, cytokine and growth factor interactions. Distinct cell types are involved in intestinal fibrosis, such as resident mesenchymal cells (fibroblasts, myofibroblasts and smooth muscle cells) but also ECM-producing cells derived from epithelial and endothelial cells (through a process termed epithelial- and endothelial-mesenchymal transition), stellate cells, pericytes, local or bone marrow-derived stem cells. The most important soluble factors that regulate the activation of these cells include cytokines, chemokines, growth factors, components of the renin-angiotensin system, angiogenic factors, peroxisome proliferator-activated receptors, mammalian target of rapamycin, and products of oxidative stress. It soon becomes clear that although inflammation is responsible for triggering the onset of the fibrotic process, it only plays a minor role in the progression of this condition, as fibrosis may advance in a self-perpetuating fashion. Definition of the cellular and molecular mechanisms involved in intestinal fibrosis may provide the key to developing new therapeutic approaches.
Keywords: Endothelial cells; Epithelial cells; Extracellular matrix; Inflammatory bowel disease; Inflammatory cells; Intestinal fibrosis; Mesenchymal cells; Molecular mediators; Myofibroblasts.
Figures
References
-
- Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10:55–60. - PubMed
-
- Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102:439–448. - PubMed
-
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

