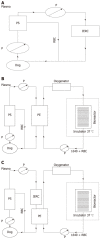
Evaluation of a novel hybrid bioartificial liver based on a multi-layer flat-plate bioreactor
- PMID: 22851870
- PMCID: PMC3406430
- DOI: 10.3748/wjg.v18.i28.3752
Evaluation of a novel hybrid bioartificial liver based on a multi-layer flat-plate bioreactor
Abstract
Aim: To evaluate the efficacy and safety of a hybrid bioartificial liver (HBAL) system in the treatment of acute liver failure.
Methods: Canine models with acute liver failure were introduced with intravenous administration of D-galactosamine. The animals were divided into: the HBAL treatment group (n = 8), in which the canines received a 3-h treatment of HBAL; the bioartificial liver (BAL) treatment group (n = 8), in which the canines received a 3-h treatment of BAL; the non-bioartificial liver (NBAL) treatment group (n = 8), in which the canines received a 3-h treatment of NBAL; the control group (n = 8), in which the canines received no additional treatment. Biochemical parameters and survival time were determined. Levels of xenoantibodies, RNA of porcine endogenous retrovirus (PERV) and reverse transcriptase (RT) activity in the plasma were detected.
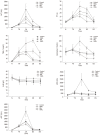
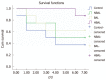
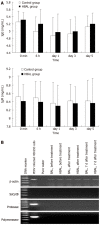
Results: Biochemical parameters were significantly decreased in all treatment groups. The TBIL level in the HBAL group was lower than that in other groups (2.19 ± 0.55 μmol/L vs 24.2 ± 6.45 μmol/L, 12.47 ± 3.62 μmol/L, 3.77 ± 1.83 μmol/L, P < 0.05). The prothrombin time (PT) in the BAL and HBAL groups was significantly shorter than the NBAL and control groups (18.47 ± 4.41 s, 15.5 ± 1.56 s vs 28.67 ± 5.71 s, 21.71 ± 3.4 s, P < 0.05), and the PT in the HBAL group was shortest of all the groups. The albumin in the BAL and HBAL groups significantly increased and a significantly higher level was observed in the HBAL group compared with the BAL group (27.7 ± 1.7 g/L vs 25.24 ± 1.93 g/L). In the HBAL group, the ammonia levels significantly decreased from 54.37 ± 6.86 to 37.75 ± 6.09 after treatment (P < 0.05); there were significant difference in ammonia levels between other the groups (P < 0.05). The levels of antibodies were similar before and after treatment. The PERV RNA and the RT activity in the canine plasma were all negative.
Conclusion: The HBAL showed great efficiency and safety in the treatment of acute liver failure.
Keywords: Acute liver failure; Co-culture; Flat plate bioreactor; Hybrid bioartificial liver; Nanofiber scaffold.
Figures
References
-
- Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. - PubMed
-
- Kinasiewicz A, Gautier A, Lewiska D, Smietanka A, Legallais C, Weryński A. Three-dimensional growth of human hepatoma C3A cells within alginate beads for fluidized bioartificial liver. Int J Artif Organs. 2008;31:340–347. - PubMed
-
- Poyck PP, van Wijk AC, van der Hoeven TV, de Waart DR, Chamuleau RA, van Gulik TM, Oude Elferink RP, Hoekstra R. Evaluation of a new immortalized human fetal liver cell line (cBAL111) for application in bioartificial liver. J Hepatol. 2008;48:266–275. - PubMed
-
- Poyck PP, Hoekstra R, van Wijk AC, Attanasio C, Calise F, Chamuleau RA, van Gulik TM. Functional and morphological comparison of three primary liver cell types cultured in the AMC bioartificial liver. Liver Transpl. 2007;13:589–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

