Retroperitoneal lymphadenectomy and resection for testicular cancer: an update on best practice
- PMID: 22852029
- PMCID: PMC3398597
- DOI: 10.1177/1756287212443170
Retroperitoneal lymphadenectomy and resection for testicular cancer: an update on best practice
Abstract
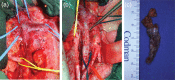
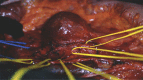
Clinical stage I testicular nonseminomatous germ cell tumours (NSGCTs) are highly curable. Following orchidectomy a risk-adapted approach using active surveillance (AS), nerve-sparing retroperitoneal lymph node dissection (nsRPLND) and primary chemotherapy is recommended by the current guidelines. Clinical stage I is defined as negative or declining tumour markers to their half-life following orchidectomy and negative imaging studies of the chest, abdomen and retroperitoneum. Active surveillance can be performed in low-risk and in high-risk NSGCTs with an anticipated relapse rate of about 15% and 50%. The majority of patients will relapse with good and intermediate prognosis tumours which have to be treated with three to four cycles chemotherapy. About 25-30% of these patients will have to undergo postchemotherapy retroperitoneal lymph node dissection (PC-RPLND) for residual masses. Primary chemotherapy with one or two cycles of cisplatin (Platinol), etoposide and bleomycin (PEB) is a therapeutic option for high-risk clinical stage I NSGCT associated with a recurrence rate of only 2-3% and a minimal acute and long-term toxicity rate. nsRPLND, if performed properly, will cure about 85% of all high-risk patients with clinical stage I NSGCT without the need for chemotherapy. PC-RPLND forms an integral part of the multimodality treatment in patients with advanced testicular germ cell tumours (TGCTs). According to current guidelines and recommendations, PC-RPLND in advanced seminomas with residual tumours is only indicated if a positron emission tomography (PET) scan performed 6-8 weeks after chemotherapy is positive. In nonseminomatous TGCT, PC-RPLND is indicated for all residual radiographic lesions with negative or plateauing markers. Loss of antegrade ejaculation represents the most common long-term complication which can be prevented by a nerve-sparing or modified template resection. The relapse rate after PC-RPLND is around 12%, however it increases significantly to about 45% in cases with redo RPLND and late relapses. Patients with increasing markers should undergo salvage chemotherapy. Only select patients with elevated markers who are thought to be chemorefractory might undergo desperation PC-RPLND if all radiographically visible lesions are completely resectable. PC-RPLND requires a complex surgical approach and should be performed in experienced, tertiary referral centres only.
Keywords: germ cell tumour; postchemotherapy RPLND; retroperitoneal lymph node dissection; retroperitoneal lymphadenectomy; testicular cancer.
Conflict of interest statement
Figures

Similar articles
-
Primary and Postchemotherapy Retroperitoneal Lymphadenectomy for Testicular Cancer.Oncol Res Treat. 2018;41(6):370-378. doi: 10.1159/000489508. Epub 2018 May 17. Oncol Res Treat. 2018. PMID: 29772568 Review.
-
Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis.Eur Urol. 2008 Feb;53(2):260-72. doi: 10.1016/j.eururo.2007.10.033. Epub 2007 Oct 31. Eur Urol. 2008. PMID: 18045770 Review.
-
Management of patients with clinical stage I nonseminomatous testicular germ cell tumours: active surveillance versus primary chemotherapy versus nerve sparing retroperitoneal lymphadenectomy.Arch Esp Urol. 2012 Mar;65(2):215-26. Arch Esp Urol. 2012. PMID: 22414450 Review. English, Spanish.
-
[Clinical outcome of postchemotherapy retroperitoneal lymph node dissection and predicting retroperitoneal histology in advanced nonseminomatous germ cell tumours of the testis].Zhonghua Wai Ke Za Zhi. 2017 Aug 1;55(8):603-607. doi: 10.3760/cma.j.issn.0529-5815.2017.08.010. Zhonghua Wai Ke Za Zhi. 2017. PMID: 28789511 Chinese.
-
Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection.Eur Urol. 2009 Jan;55(1):217-24. doi: 10.1016/j.eururo.2008.09.027. Epub 2008 Sep 24. Eur Urol. 2009. PMID: 18926622
Cited by
-
Unilateral post-chemotherapy robot-assisted retroperitoneal lymph node dissection in Stage II non-seminomatous germ cell tumor: A tertiary care experience.Asian J Urol. 2023 Oct;10(4):440-445. doi: 10.1016/j.ajur.2023.05.002. Epub 2023 Jul 25. Asian J Urol. 2023. PMID: 38024429 Free PMC article.
-
Predictive capacity of a miRNA panel in identifying teratoma in post-chemotherapy consolidation surgeries.BJUI Compass. 2022 Jul 5;4(1):81-87. doi: 10.1002/bco2.143. eCollection 2023 Jan. BJUI Compass. 2022. PMID: 36569509 Free PMC article.
-
[Post-chemotherapy laparoscopic retroperitoneal lymph node dissection in low volume residual germ cell cancer: a technique to reduce morbidity].Urologe A. 2013 Aug;52(8):1097-103. doi: 10.1007/s00120-013-3133-5. Urologe A. 2013. PMID: 23416965 Clinical Trial. German.
-
Trans- and extraperitoneal retroperitoneal lymph node dissection (RPLND) in the treatment for nonseminomatous germ cell testicular tumors (NSGCT): a single Chinese center's retrospective analysis.Int Urol Nephrol. 2014 Feb;46(2):363-9. doi: 10.1007/s11255-013-0547-3. Epub 2013 Sep 1. Int Urol Nephrol. 2014. PMID: 23996573
-
Chylous Ascites and Lymphoceles: Evaluation and Interventions.Semin Intervent Radiol. 2020 Aug;37(3):274-284. doi: 10.1055/s-0040-1713445. Epub 2020 Jul 31. Semin Intervent Radiol. 2020. PMID: 32773953 Free PMC article. Review.
References
-
- Albers P., Albrecht W., Algaba F., Bokemeyer C., Cohn-Cedermark G., Horwich A., et al. (2005) Guidelines on testicular cancer. Eur Urol 48: 885–894 - PubMed
-
- Albers P., Ganz A., Hannig E., Miersch W.D., Müller S.C. (2000) Salvage surgery of chemorefractory germ cell tumors with elevated markers. J Urol 164: 381–384 - PubMed
-
- Albers P., Höltl W., Heidenreich A., Aharinejad S. (2004a) Thoracoabdominal resection of retrocrural residual tumors. Akt Urol 35: 141–150 - PubMed
-
- Albers P., Melchior D., Müller S.C. (2003a) Extensive surgery in testis cancer. Eur Urol 44: 233–244 - PubMed
-
- Albers P., Siener R., Kliesch S., Weissbach L., Krege S., Sparwasser C., et al. for the German Testicular Cancer Study Group (2003a) Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol 21: 1505–1512 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

