Influenza virus-mediated membrane fusion: determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion
- PMID: 22852045
- PMCID: PMC3407899
- DOI: 10.3390/v4071144
Influenza virus-mediated membrane fusion: determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion
Abstract
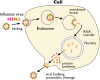
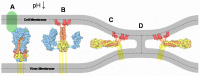
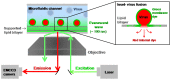
Hemagglutinin (HA) is the viral protein that facilitates the entry of influenza viruses into host cells. This protein controls two critical aspects of entry: virus binding and membrane fusion. In order for HA to carry out these functions, it must first undergo a priming step, proteolytic cleavage, which renders it fusion competent. Membrane fusion commences from inside the endosome after a drop in lumenal pH and an ensuing conformational change in HA that leads to the hemifusion of the outer membrane leaflets of the virus and endosome, the formation of a stalk between them, followed by pore formation. Thus, the fusion machinery is an excellent target for antiviral compounds, especially those that target the conserved stem region of the protein. However, traditional ensemble fusion assays provide a somewhat limited ability to directly quantify fusion partly due to the inherent averaging of individual fusion events resulting from experimental constraints. Inspired by the gains achieved by single molecule experiments and analysis of stochastic events, recently-developed individual virion imaging techniques and analysis of single fusion events has provided critical information about individual virion behavior, discriminated intermediate fusion steps within a single virion, and allowed the study of the overall population dynamics without the loss of discrete, individual information. In this article, we first start by reviewing the determinants of HA fusogenic activity and the viral entry process, highlight some open questions, and then describe the experimental approaches for assaying fusion that will be useful in developing the most effective therapies in the future.
Keywords: hemagglutinin; influenza virus; membrane fusion; protease; virus entry.
Figures

References
-
- Horimoto T., Kawaoka Y. Influenza: Lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 2005;3:591–600. - PubMed
-
- Palese P., Shaw M.L. Orthomyxoviridae: The Viruses and Their Replication. In: Knipe D.M., editor. Fields Virology. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2007.
-
- Nelson M.I., Holmes E.C. The evolution of epidemic influenza. Nat. Rev. Genet. 2007;8:196–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

