Bacillus anthracis edema factor substrate specificity: evidence for new modes of action
- PMID: 22852066
- PMCID: PMC3407890
- DOI: 10.3390/toxins4070505
Bacillus anthracis edema factor substrate specificity: evidence for new modes of action
Abstract
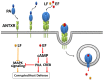
Since the isolation of Bacillus anthracis exotoxins in the 1960s, the detrimental activity of edema factor (EF) was considered as adenylyl cyclase activity only. Yet the catalytic site of EF was recently shown to accomplish cyclization of cytidine 5'-triphosphate, uridine 5'-triphosphate and inosine 5'-triphosphate, in addition to adenosine 5'-triphosphate. This review discusses the broad EF substrate specificity and possible implications of intracellular accumulation of cyclic cytidine 3':5'-monophosphate, cyclic uridine 3':5'-monophosphate and cyclic inosine 3':5'-monophosphate on cellular functions vital for host defense. In particular, cAMP-independent mechanisms of action of EF on host cell signaling via protein kinase A, protein kinase G, phosphodiesterases and CNG channels are discussed.
Keywords: Bacillus anthracis; adenylyl cyclase toxin; anthrax; edema factor; edema toxin.
Figures
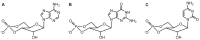

Similar articles
-
Cytidylyl and uridylyl cyclase activity of bacillus anthracis edema factor and Bordetella pertussis CyaA.Biochemistry. 2010 Jul 6;49(26):5494-503. doi: 10.1021/bi100684g. Biochemistry. 2010. PMID: 20521845 Free PMC article.
-
The adenylyl cyclase activity of anthrax edema factor.Mol Aspects Med. 2009 Dec;30(6):423-30. doi: 10.1016/j.mam.2009.06.001. Epub 2009 Jun 26. Mol Aspects Med. 2009. PMID: 19560485 Free PMC article. Review.
-
Different Roles of N-Terminal and C-Terminal Domains in Calmodulin for Activation of Bacillus anthracis Edema Factor.Toxins (Basel). 2015 Jul 13;7(7):2598-614. doi: 10.3390/toxins7072598. Toxins (Basel). 2015. PMID: 26184312 Free PMC article.
-
Anthrax Edema Factor: An Ion-Adaptive Mechanism of Catalysis with Increased Transition-State Conformational Flexibility.J Phys Chem B. 2016 Jul 14;120(27):6504-14. doi: 10.1021/acs.jpcb.6b02527. Epub 2016 Jun 24. J Phys Chem B. 2016. PMID: 27260163
-
The adenylate cyclase toxins.Crit Rev Microbiol. 2004;30(3):187-96. doi: 10.1080/10408410490468795. Crit Rev Microbiol. 2004. PMID: 15490970 Review.
Cited by
-
Nucleotidyl cyclase activity of particulate guanylyl cyclase A: comparison with particulate guanylyl cyclases E and F, soluble guanylyl cyclase and bacterial adenylyl cyclases CyaA and edema factor.PLoS One. 2013 Jul 29;8(7):e70223. doi: 10.1371/journal.pone.0070223. Print 2013. PLoS One. 2013. PMID: 23922959 Free PMC article.
-
A Simple Luminescent Adenylate-Cyclase Functional Assay for Evaluation of Bacillus anthracis Edema Factor Activity.Toxins (Basel). 2016 Aug 18;8(8):243. doi: 10.3390/toxins8080243. Toxins (Basel). 2016. PMID: 27548219 Free PMC article.
-
Diminished but Not Abolished Effect of Two His351 Mutants of Anthrax Edema Factor in a Murine Model.Toxins (Basel). 2016 Feb 2;8(2):35. doi: 10.3390/toxins8020035. Toxins (Basel). 2016. PMID: 26848687 Free PMC article.
-
Group 3 innate lymphocytes (ILC3s) upregulate IL-22 in response to elevated intracellular cAMP levels.Cytokine. 2022 May;153:155862. doi: 10.1016/j.cyto.2022.155862. Epub 2022 Mar 17. Cytokine. 2022. PMID: 35306427 Free PMC article.
-
Modulation of innate lymphoid cells by enteric bacterial pathogens.Front Immunol. 2023 Jul 6;14:1219072. doi: 10.3389/fimmu.2023.1219072. eCollection 2023. Front Immunol. 2023. PMID: 37483638 Free PMC article. Review.
References
-
- Defer N., Best-Belpomme M., Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am. J. Physiol. Renal Physiol. 2000;279:F400–F416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

