Osmotic drug delivery system as a part of modified release dosage form
- PMID: 22852100
- PMCID: PMC3407637
- DOI: 10.5402/2012/528079
Osmotic drug delivery system as a part of modified release dosage form
Abstract
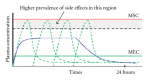
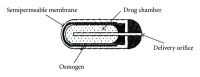
Conventional drug delivery systems are known to provide an immediate release of drug, in which one can not control the release of the drug and can not maintain effective concentration at the target site for longer time. Controlled drug delivery systems offer spatial control over the drug release. Osmotic pumps are most promising systems for controlled drug delivery. These systems are used for both oral administration and implantation. Osmotic pumps consist of an inner core containing drug and osmogens, coated with a semipermeable membrane. As the core absorbs water, it expands in volume, which pushes the drug solution out through the delivery ports. Osmotic pumps release drug at a rate that is independent of the pH and hydrodynamics of the dissolution medium. The historical development of osmotic systems includes development of the Rose-Nelson pump, the Higuchi-Leeper pumps, the Alzet and Osmet systems, the elementary osmotic pump, and the push-pull system. Recent advances include development of the controlled porosity osmotic pump, and systems based on asymmetric membranes. This paper highlights the principle of osmosis, materials used for fabrication of pumps, types of pumps, advantages, disadvantages, and marketed products of this system.
Figures










Similar articles
-
Osmotic Pump Drug Delivery Systems-A Comprehensive Review.Pharmaceuticals (Basel). 2022 Nov 18;15(11):1430. doi: 10.3390/ph15111430. Pharmaceuticals (Basel). 2022. PMID: 36422560 Free PMC article. Review.
-
Recent advances in asymmetric membrane capsule based osmotic pump: a patent overview.Recent Pat Drug Deliv Formul. 2012 Apr 1;6(1):66-72. doi: 10.2174/187221112799219099. Recent Pat Drug Deliv Formul. 2012. PMID: 22191697 Review.
-
Modified push-pull osmotic system for simultaneous delivery of theophylline and salbutamol: development and in vitro characterization.Int J Pharm. 2004 Oct 13;284(1-2):95-108. doi: 10.1016/j.ijpharm.2004.07.015. Int J Pharm. 2004. PMID: 15454301
-
Development and evaluation of push-pull based osmotic delivery system for pramipexole.PDA J Pharm Sci Technol. 2008 Jan-Feb;62(1):22-31. PDA J Pharm Sci Technol. 2008. PMID: 18402365
-
Recent Aspects of Osmotic Pump Systems: Functionalization, Clinical use and Advanced Imaging Technology.Curr Drug Metab. 2016;17(3):279-91. doi: 10.2174/1389200216666151015115706. Curr Drug Metab. 2016. PMID: 26467064 Review.
Cited by
-
Development of a Model Based on Physical Mechanisms for the Explanation of Drug Release: Application to Diclofenac Release from Polyurethane Films.Polymers (Basel). 2021 Apr 10;13(8):1230. doi: 10.3390/polym13081230. Polymers (Basel). 2021. PMID: 33920267 Free PMC article.
-
Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases.Biomed Microdevices. 2019 May 18;21(2):47. doi: 10.1007/s10544-019-0389-6. Biomed Microdevices. 2019. PMID: 31104136 Free PMC article. Review.
-
Quality by Design (QbD)-Based Numerical and Graphical Optimization Technique for the Development of Osmotic Pump Controlled-Release Metoclopramide HCl Tablets.Drug Des Devel Ther. 2020 Nov 26;14:5217-5234. doi: 10.2147/DDDT.S278918. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33273807 Free PMC article.
-
Polymer Delivery Systems for Long-Acting Antiretroviral Drugs.Pharmaceutics. 2024 Jan 28;16(2):183. doi: 10.3390/pharmaceutics16020183. Pharmaceutics. 2024. PMID: 38399244 Free PMC article. Review.
-
Osmotic Pump Drug Delivery Systems-A Comprehensive Review.Pharmaceuticals (Basel). 2022 Nov 18;15(11):1430. doi: 10.3390/ph15111430. Pharmaceuticals (Basel). 2022. PMID: 36422560 Free PMC article. Review.
References
-
- Prescott LF. Novel Drug Delivery and Its Therapeutic application . West Susset, UK: John Wiley and Sons; 1989. The need for improved drug delivery in clinical practice; pp. 1–11.
-
- Verma RK, Garg S. Current status of drug delivery technologies and future directions. Pharmaceutical Technology . 2001;25(2):1–14.
-
- Dwarakanadha Reddy P, Swarnalatha D. Recent Advances in Novel Drug Delivery systems. International Journal of PharmTech Research . 2010;2(3):2025–2027.
-
- Rastogi SK, Vaya N, Mishra B. Osmotic pump: a novel concept in rate controlled oral drug delivery. Eastern Pharmacist . 1995;38:79–82.
-
- Banker RW. Control Release of Biologically Active Agent . New York, NY, USA: John Wiley and Son; 1987.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

