Upregulation of transmitter release probability improves a conversion of synaptic analogue signals into neuronal digital spikes
- PMID: 22852823
- PMCID: PMC3497613
- DOI: 10.1186/1756-6606-5-26
Upregulation of transmitter release probability improves a conversion of synaptic analogue signals into neuronal digital spikes
Abstract
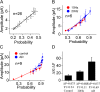
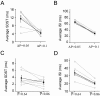
Action potentials at the neurons and graded signals at the synapses are primary codes in the brain. In terms of their functional interaction, the studies were focused on the influence of presynaptic spike patterns on synaptic activities. How the synapse dynamics quantitatively regulates the encoding of postsynaptic digital spikes remains unclear. We investigated this question at unitary glutamatergic synapses on cortical GABAergic neurons, especially the quantitative influences of release probability on synapse dynamics and neuronal encoding. Glutamate release probability and synaptic strength are proportionally upregulated by presynaptic sequential spikes. The upregulation of release probability and the efficiency of probability-driven synaptic facilitation are strengthened by elevating presynaptic spike frequency and Ca2+. The upregulation of release probability improves spike capacity and timing precision at postsynaptic neuron. These results suggest that the upregulation of presynaptic glutamate release facilitates a conversion of synaptic analogue signals into digital spikes in postsynaptic neurons, i.e., a functional compatibility between presynaptic and postsynaptic partners.
Figures
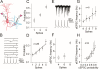





Similar articles
-
Gain and fidelity of transmission patterns at cortical excitatory unitary synapses improve spike encoding.J Cell Sci. 2008 Sep 1;121(Pt 17):2951-60. doi: 10.1242/jcs.025684. Epub 2008 Aug 12. J Cell Sci. 2008. PMID: 18697836
-
Synaptic structural complexity as a factor enhancing probability of calcium-mediated transmitter release.J Neurophysiol. 1996 Jun;75(6):2451-66. doi: 10.1152/jn.1996.75.6.2451. J Neurophysiol. 1996. PMID: 8793756
-
An algorithm for modifying neurotransmitter release probability based on pre- and postsynaptic spike timing.Neural Comput. 2001 Jan;13(1):35-67. doi: 10.1162/089976601300014628. Neural Comput. 2001. PMID: 11177427
-
Ca2+-dependent mechanisms of presynaptic control at central synapses.Neuroscientist. 2006 Aug;12(4):317-26. doi: 10.1177/1073858405284672. Neuroscientist. 2006. PMID: 16840708 Free PMC article. Review.
-
Calcium and transmitter release.J Physiol Paris. 1993;87(1):25-36. doi: 10.1016/0928-4257(93)90021-k. J Physiol Paris. 1993. PMID: 7905762 Review.
Cited by
-
GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression.Oncotarget. 2017 May 30;8(22):35933-35945. doi: 10.18632/oncotarget.16411. Oncotarget. 2017. PMID: 28415589 Free PMC article.
-
Homeostatic synaptic plasticity rescues neural coding reliability.Nat Commun. 2023 May 24;14(1):2993. doi: 10.1038/s41467-023-38575-6. Nat Commun. 2023. PMID: 37225688 Free PMC article.
-
PKC and CaMK-II inhibitions coordinately rescue ischemia-induced GABAergic neuron dysfunction.Oncotarget. 2017 Jun 13;8(24):39309-39322. doi: 10.18632/oncotarget.16947. Oncotarget. 2017. PMID: 28445148 Free PMC article.
-
Functional compatibility between Purkinje cell axon branches and their target neurons in the cerebellum.Oncotarget. 2017 Aug 1;8(42):72424-72437. doi: 10.18632/oncotarget.19770. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 29069799 Free PMC article.
-
Cortical GABAergic neurons are more severely impaired by alkalosis than acidosis.BMC Neurol. 2013 Dec 5;13:192. doi: 10.1186/1471-2377-13-192. BMC Neurol. 2013. PMID: 24314112 Free PMC article.
References
-
- Kandel ER, Siegelbaum SA, Schwartz JH. In: Principles of Neural Science. Kandel ER, Schwartz JH, Jessell TM, editor. New York: McGraw-Hill; 2000. Elementary interactions between neurons: Synaptic transmission; pp. 175–308.
-
- Shepherd GM. In: Neurobiology. Shepherd GM, editor. New York: Oxford University Press; 1998. Synaptic transmission.
-
- Byrne JH. Learning and Memory: Basic Mechanisms. Amsterdam: Academic Press; 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

